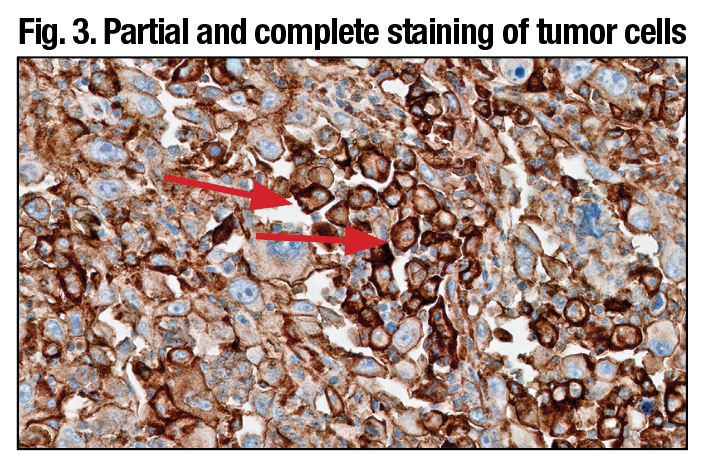
PD-L1 primary antibody exhibiting linear membrane staining distinct from cytoplasmic staining (arrows) (20× magnification).
However, Dr. Hanks emphasizes, it’s important to keep in mind that when the first impression is that the CPS is less than one, “it’s not enough to determine if your specimen is indeed CPS zero or PD-L1 expression-negative from a low power scan. You have to review the entire slide at 20× to make sure you’re not missing pockets of weakly staining tumor cells or lymphocytes or macrophages.”
The CPS exclusion criteria specifically rule out any PD-L1-positive immune cells associated with adenoma, dysplasia, carcinoma, or with ulcers, chronic gastritis, or other processes not associated with the tumor, Dr. Hanks says. “We look at the H&E stain slide of patients’ tumors very carefully, and pathologists are able to identify where the tumor cells are versus where adenoma or carcinoma in situ are located.”
“We do not count immune cells that are associated with normal structures, and we do not include neutrophils, eosinophils, or plasma cells. In gastric cancer, you can find small clusters of plasma cells and they tend to stain with a very weak cytoplasmic blush. They would be excluded from scoring.”
 Ganglion cells reflect another ailment that is excluded. These can be found deep in the smooth muscle wall of a gastric resection specimen and can have a blush stain that is similar to cytoplasmic or membrane staining, but should not be counted. However, “We have also found that a low-power streaming of staining could be interpreted as only fibroblast staining, but when you get to 20× there are some immune cells mixed within fibroblasts.” Also excluded are stromal cells including fibroblasts and, naturally, necrotic cells or cellular debris. Sample images in Agilent literature on the PD-L1 assay, including the interpretation manual and E-Learning modules, explain this aspect of the CPS in greater detail.
Ganglion cells reflect another ailment that is excluded. These can be found deep in the smooth muscle wall of a gastric resection specimen and can have a blush stain that is similar to cytoplasmic or membrane staining, but should not be counted. However, “We have also found that a low-power streaming of staining could be interpreted as only fibroblast staining, but when you get to 20× there are some immune cells mixed within fibroblasts.” Also excluded are stromal cells including fibroblasts and, naturally, necrotic cells or cellular debris. Sample images in Agilent literature on the PD-L1 assay, including the interpretation manual and E-Learning modules, explain this aspect of the CPS in greater detail.
Evaluation of the CPS denominator involves a process pathologists are familiar with from scoring estrogen and progesterone receptors, Dr. Hanks says. With CPS, the task is somewhat more quantitative. “All the viable tumor cells on your slide count, so tumor cells with and without staining should be carefully examined to precisely assess the denominator.” For example, with a tumor cell that’s about 20 µm in diameter, there are about 2,500 cells filling a typical 20× field.

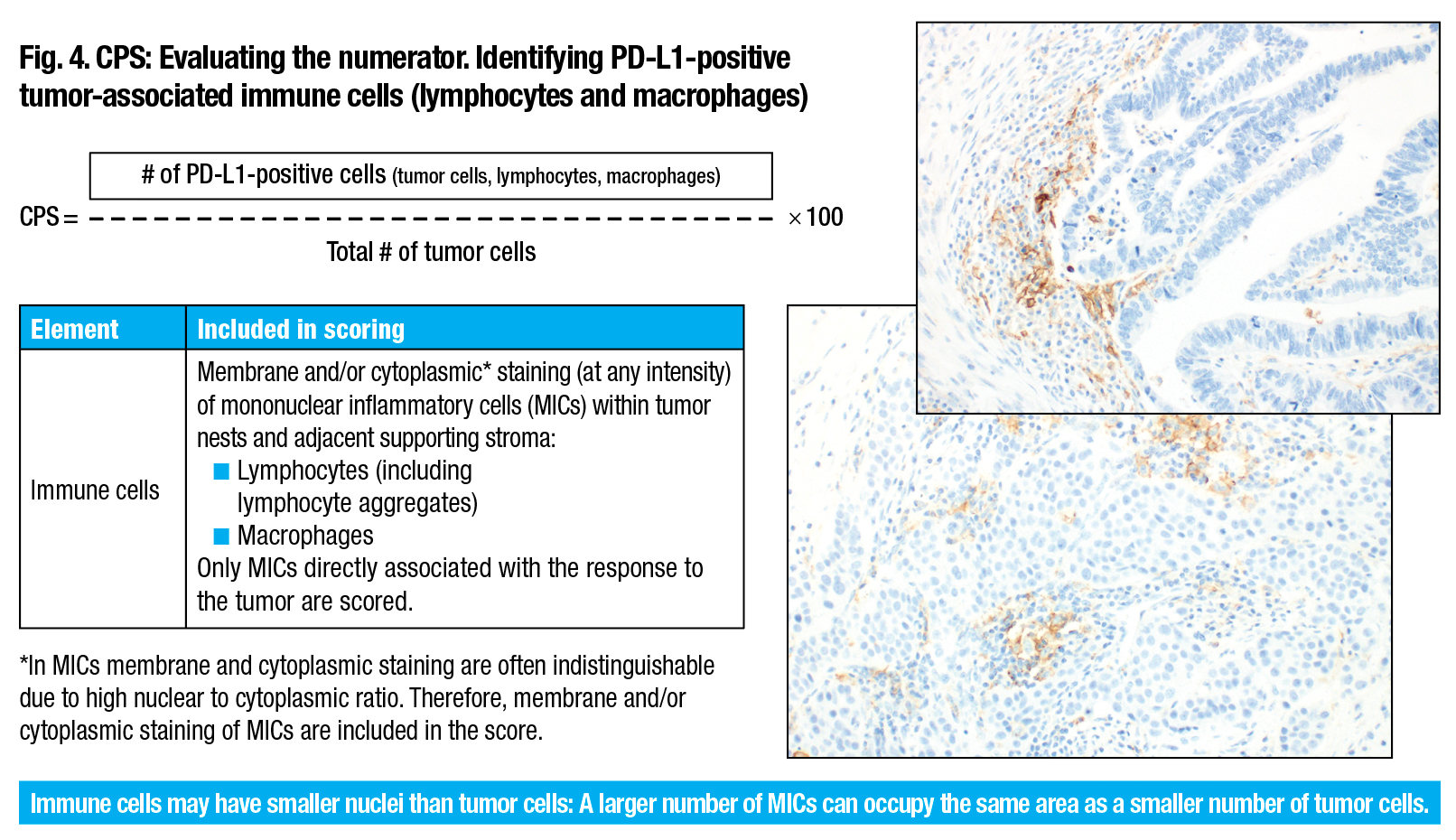
PD-L1 primary antibody exhibiting linear membrane and/or cytoplasmic staining of tumor-associated mononuclear inflammatory cells (arrows) (20× magnification).
Diffuse gastric carcinoma creates other difficulties for pathologists evaluating the denominator, Dr. Hanks notes. “Those pathologists who frequently look at resections know there can be a dense infiltrate right underneath the mucosa, and then the cells invade through the smooth muscle to the point where it may be a slight challenge to distinguish between smooth muscle, fibroblasts, and carcinoma cells. And then the cells may even be denser at the serosal surface.” The space between tumor cells can make the specimen appear much less cellular than a solid tumor involving the same area. But tumor cells may blend into the background, so the specimen may be more cellular than it appears at first glance.
The combined positive score is appropriate for analyzing metastatic gastric cancer to a lymph node, Dr. Hanks says, but the magnification level is important. “Obviously, lymph nodes can have a low level of PD-L1 positivity in adjacent normal lymphoid tissue, so the 20× field recommendation is applied in lymph node.” Agilent’s E-Learning module provides examples of this application of the scoring method.
Scoring strategies provided by Agilent also include approaches that can be used with one patch or multiple patches of positivity, Dr. Hanks notes. “If you have a sea of negativity and one small patch that’s a CPS of one, obviously your overall score is CPS zero or negative—no PD-L1 expression.”
 “If you have, as in this case (Fig. 6), a point where there is a patch where the CPS is actually 80, and all of the rest of it is negative and it represents 10 percent of your specimen, the math works out that the CPS is eight, so this specimen is PD-L1 positive.”
“If you have, as in this case (Fig. 6), a point where there is a patch where the CPS is actually 80, and all of the rest of it is negative and it represents 10 percent of your specimen, the math works out that the CPS is eight, so this specimen is PD-L1 positive.”
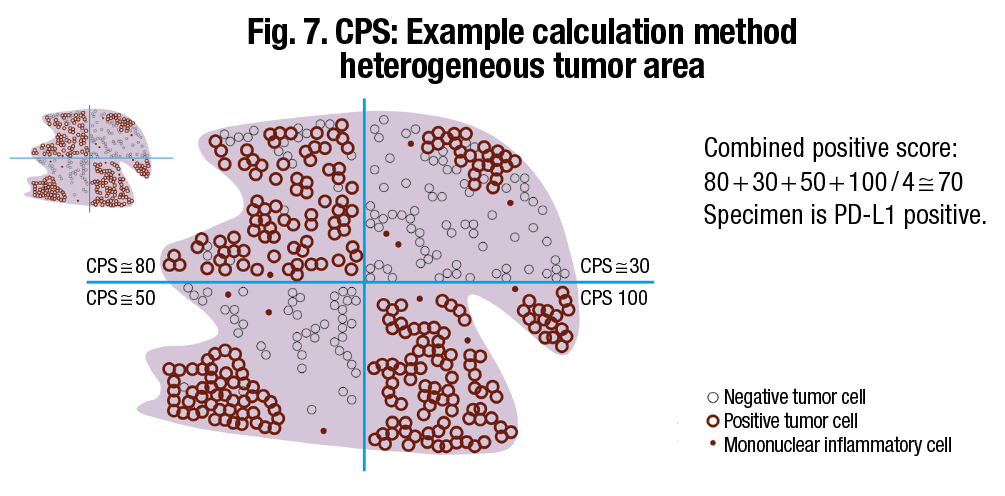
Agilent offers a “divide and conquer” strategy for scoring large resection specimens, which involves dividing a large section of tissue into four equal quadrants and solving by sector, Dr. Hanks says. “You would divide the tissue up so there are approximately an equal number of cells in each quadrant, then add up the scores and divide by four.” (Fig. 7).
A common mistake is to underestimate the denominator in the CPS by underestimating the number of unstained tumor cells, resulting in a falsely elevated CPS and possibly a mistaken eligibility for anti-PD-1 treatment. Similarly, if the denominator is overcounted, the patient is at risk of not qualifying for treatment. The only way to avoid such errors is to practice the method, Dr. Hanks emphasizes.
 Combined positive scores are reliable, especially around the critical cutoff of one, in determining PD-L1 expression or no PD-L1 expression, she says. Agilent has found, in analyzing scores across instruments, platforms, and pathologists, that consistency is also high. “CPS in gastric cancer passed all statistical criteria for solid reproducibility and intra-observer variability. After training and practice, pathologists can align with this algorithm and can reproducibly and reliably score gastric cancer specimens at the CPS ≥1.”
Combined positive scores are reliable, especially around the critical cutoff of one, in determining PD-L1 expression or no PD-L1 expression, she says. Agilent has found, in analyzing scores across instruments, platforms, and pathologists, that consistency is also high. “CPS in gastric cancer passed all statistical criteria for solid reproducibility and intra-observer variability. After training and practice, pathologists can align with this algorithm and can reproducibly and reliably score gastric cancer specimens at the CPS ≥1.”
As a general precept, “we recommend that evaluation of PD-L1 stains be performed within the context of the pathologist’s past experience and best judgment in interpreting IHC stains.” However, the most difficult part of a diagnostic workup using the PD-L1 assay in gastric cancer is the CPS, she believes. “It takes time to see a few cases to get a low-power gestalt. Are you scoring something that’s obviously positive with a high CPS versus obviously a zero? Those are the easy cases.”
Since each case is different, each may require a different strategy, and different pathologists may prefer one method over another, Dr. Hanks points out. “We’re finding that pathologists have different ways to approach cases and they find what works for them.” But some of her advice applies to all pathologists who wish to avoid problems with complex cases. “Make sure you see all of the positive and all of the negative. Have you considered the denominator? After doing your combined positive score calculation, review the slide. Does it make sense? Can you defend your score? Can you reproduce your score? And practice, practice, practice.”
As with any companion diagnostic with a cutoff score, more time is spent around the cutoff. “In my experience, when I am near that CPS of one with a gastric cancer case, whether it’s intestinal or diffuse pattern, I know I’m going to have to spend a little more time. It may take me 10 minutes to sit and make sure I’ve looked at all the positives and all the negatives to have an estimation of what the denominator is before I make the decision.”
How results are reported will vary depending on the laboratory’s information system, Dr. Hanks notes. An example of how to report results is included in the interpretation manual and in principle four of the first E-Learning module. “The FDA required that our example include how many biopsies were actually obtained and have that in the report. In addition,” she says, “the report shows the CPS score and whether the sample result is interpreted with PD-L1 expression or no PD-L1 expression.”
“There is a full page of other details that we suggest should be included.” Among these are the type of tissue, number of biopsies in the tissue block, and check-offs covering control cell line slide results and the adequacy of tumor cells present. However, Dr. Hanks says, “Including a lot of this information is really the final decision of the laboratory director or the medical director of the lab.”
In response to questions about use of different platforms to bring Agilent’s PD-L1 IHC test online, Dr. Eklund cautions that Agilent does not endorse off-label use of its products. “If users are modifying any part of the validated full solution approved by the FDA, it represents a laboratory-developed test, for which users are responsible for conducting a full validation according to country-specific recommendations. Different organizations globally have published recommendations on validation of IVD devices, but ultimately it is the responsibility of the medical director in the laboratory to ensure that the tests they are using are fit for the purpose.”
The CPS process is not only valuable but also relatively easy to learn, Dr. Hanks says. Agilent conducted training in a pre-commercial setting and found that pathologists in private practice who were trained performed just as well at scoring the CPS as they did with the tumor proportion score.
“Pathologists may wonder if this can be applied in their practice and I think it’s important for them to take time to look at the E-Learning modules and get practice with the algorithms. Pathologists whom we have trained catch on to scoring with CPS very quickly, and even those who haven’t scored using TPS in lung cancer in the past also catch on. It’s just a matter of trying it—and practicing.”
[hr]
Anne Paxton is a writer and attorney in Seattle. The Agilent E-Learning modules are at www.agilent.com/en-us/e-learning-dako-products. Log-in is required.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
