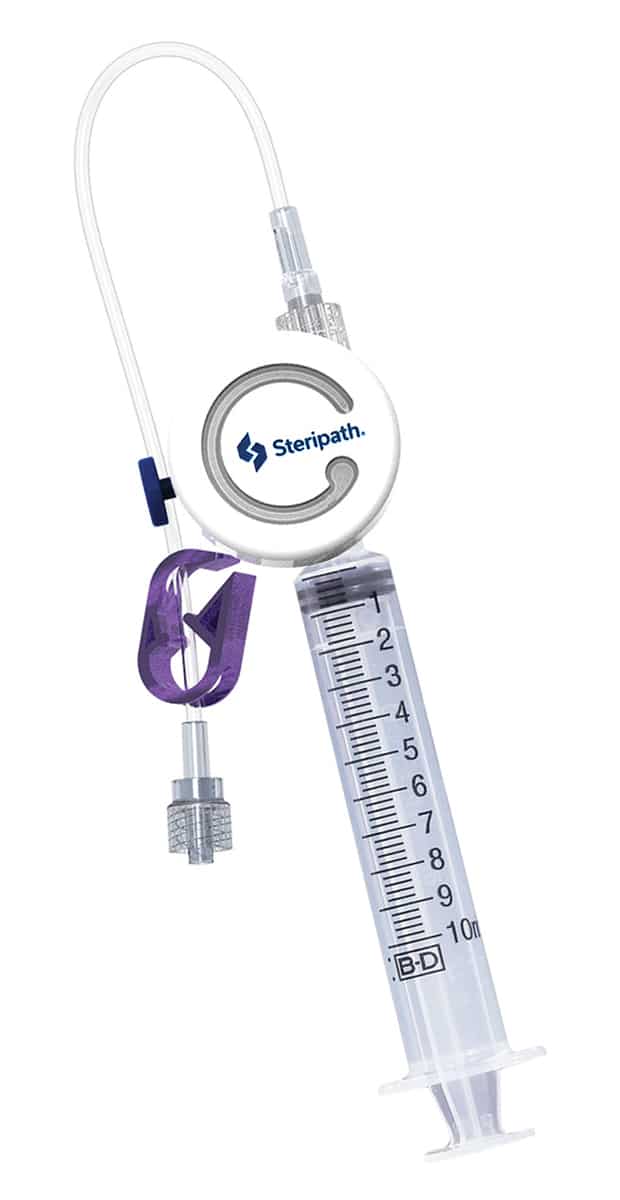
 October 2021—Magnolia Medical Technologies announced the commercial availability of its Steripath Micro Initial Specimen Diversion Device designed for use in children’s hospitals. Steripath Micro is an FDA 510(k)-cleared device with the specific indication to reduce blood culture contamination. The small, lightweight device requires less than 1 mL of blood for patients with limited volumes or difficult intravenous access. The company says Steripath Micro has shown a zero percent contamination rate in two leading children’s hospitals during a three- month period.
October 2021—Magnolia Medical Technologies announced the commercial availability of its Steripath Micro Initial Specimen Diversion Device designed for use in children’s hospitals. Steripath Micro is an FDA 510(k)-cleared device with the specific indication to reduce blood culture contamination. The small, lightweight device requires less than 1 mL of blood for patients with limited volumes or difficult intravenous access. The company says Steripath Micro has shown a zero percent contamination rate in two leading children’s hospitals during a three- month period.
Pages: 1 2
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
