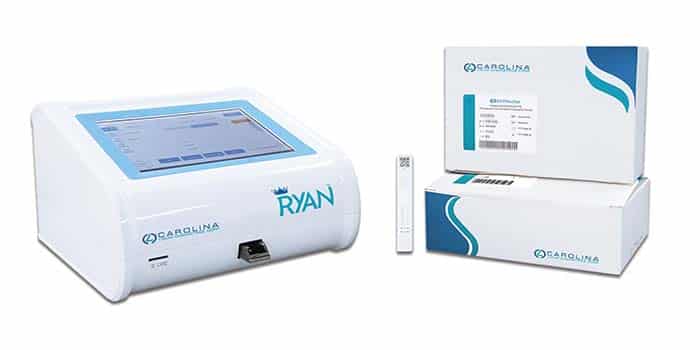
April 2023—Shenzhen Superbio Technology has received FDA clearance for the Ryan immunofluorescence analyzer, a point-of-care instrument for in vitro diagnostic use only. Carolina Liquid Chemistries Corp. will distribute the analyzer and fentanyl urine detection kit in partnership with Bioeasy USA. The fentanyl detection kit is intended for the qualitative detection of fentanyl in human urine at a cutoff concentration of 1 ng/mL. It is intended for use with the Ryan analyzer, which provides results in less than six minutes.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management