Amy Carpenter Aquino
April 2019—Diagnoses of essential thrombocythemia and chronic myelomonocytic leukemia were among those covered in four cases in a CAP18 session on practical challenges in peripheral blood evaluation.
Carla S. Wilson, MD, PhD, professor of hematopathology, and Devon S. Chabot-Richards, MD, associate professor of hematopathology and molecular pathology, Department of Pathology, University of New Mexico School of Medicine, walked attendees through seven cases illustrating the benefits of peripheral blood smear evaluation, four of which are reported here. Drs. Wilson and Chabot-Richards are medical directors at TriCore Reference Laboratories, Albuquerque, NM.
The first case was that of a 58-year-old man who presented with a three-month history of weakness, palpitations, and shortness of breath. He complained of bleeding gums, epistaxis, and a tingling sensation throughout his body. His CBC with differential counts revealed marked anemia (Hb 5.6 gm/dL), high RDW (21.2 percent), mild leukopenia (WBC 2.7 × 109/L), lymphopenia, adequate neutrophils, and moderate thrombocytopenia (platelet 68 × 109/L).
Review of the peripheral blood smear was remarkable for prominent red cell anisopoikilocytosis with schistocytes, oval macrocytes, rare spherocytes, and nonspecific poikilocytes. Polychromasia was not increased and the reticulocyte count was normal, Dr. Wilson said.
Additional studies revealed that the patient had low haptoglobin (<8 mg/dL), elevated LDH (1023 U/L), and elevated indirect bilirubin (3.5; normal range 0.2–1.0) consistent with hemolysis, she said. “We recommended additional testing which showed a serum vitamin B12 level of <60 pg/mL [normal 193–986], normal RBC folate level, methylmalonic acid of 3.08 µmol/L [normal 0–0.4], and positive intrinsic factor and parietal cell antibodies. The clinicians were worried about thrombotic thrombocytopenic purpura because of all the schistocytes,” Dr. Wilson said. They sent out for an ADAMTS13 activity assay; results were normal.
“This is a case of severe vitamin B12 deficiency that can mimic microangiopathic hemolytic anemia,” or MAHA, she said. “The key is that you can see a lot of fragmented red cells in vitamin B12 deficiency due to increased red cell fragility causing decreased survival in circulation.” (Fig. 1)
Severe vitamin B12 deficiency affects DNA synthesis in all hematopoietic lineages, and the CBC often shows pancytopenia.
Hypersegmented neutrophils are an important clue to vitamin B12 deficiency, as shown in Fig. 1. Hypersegmentation is defined as the presence of six or more lobes in a neutrophil or five lobes in at least five percent of neutrophils. This abnormality is quickly reversed and may not be recognized in smears after initiation of vitamin B12 supplementation. Hypersegmented neutrophils are not specific to vitamin B12 deficiency. “You can see hypersegmented neutrophils and macrocytic anemia in association with myelodysplastic syndromes or in individuals receiving certain drugs such as hydroxyurea,” Dr. Wilson said.
“How do we distinguish vitamin B12 deficiency from microangiopathic hemolytic anemia?” she asked.
- A high reticulocyte count can be a reliable indicator of MAHA, she said. The reticulocyte percentage may be elevated in B12 deficiency but the absolute reticulocyte number is low due to ineffective hematopoiesis.
- Pancytopenia is rare in MAHA. The degree of anemia is not as severe in MAHA and platelet counts are typically lower than in B12 deficiency.
- The MCV in vitamin B12 deficiency is usually high and often exceeds 120 fl, although it may be normal in cases of severe hemolysis or with concurrent iron deficiency or thalassemia. Reticulocytosis associated with MAHA only causes a slightly elevated MCV, if at all. The MCV was 114 fl in this case.
- Neutrophil hypersegmentation or neutropenia is not a feature of MAHA.
 “The problem with making a diagnosis of vitamin B12 deficiency in less severe cases is that no single marker detects deficiency in all patients,” she said, and serum vitamin B12 testing is neither sensitive nor specific.
“The problem with making a diagnosis of vitamin B12 deficiency in less severe cases is that no single marker detects deficiency in all patients,” she said, and serum vitamin B12 testing is neither sensitive nor specific.
Reference ranges for serum vitamin B12 are based on population studies and may not be adequate for individuals. Another problem is that serum B12 assays measure the total B12 bound to both transcobalamin (20 percent) and haptocorrin (80 percent). Only the 20 percent of B12 bound to transcobalamin is available to tissues through CD320 cell receptors. The 80 percent bound to haptocorrin is metabolically unavailable; serum B12 measurements are significantly affected by changes in the haptocorrin protein level. Increased haptocorrin is produced by the liver in some malignancies, and about 15 percent of people normally have decreased levels.
The metabolically active B12-transcobalamin complex is called holotranscobalamin (HTC), and some laboratories, particularly those outside the United States, are testing for HTC rather than total serum B12 as a measure of B12 deficiency.
“With decreased vitamin B12, you see decreased HTC,” Dr. Wilson said. “There is less uptake of B12 into tissues, and it starts affecting the B12 dependent pathways.”
The immunoassay for HTC is being marketed as an active B12 assay, and preliminary studies suggest it may have some of the same problems as the existing serum B12 assays, most notably when the HTC results fall into an indeterminate range of 25 to 70 pmol/L (depending on the assay). Similar to serum B12 assays, results in the intermediate range should be followed up with tests for methylmalonic acid (MMA) preferentially or homocysteine.
MMA is an imperfect test for some individuals, Dr. Wilson said, and particularly for patients with renal disease who do not excrete serum metabolites of MMA. The elevated metabolites can make the patient appear to be B12 deficient. “MMA is better than homocysteine because it’s usually not elevated in folate deficiency and homocysteine is also affected by renal disease,” she said. Automated tests for MMA can detect subtle disturbances in vitamin B12 metabolic pathways and should be combined with serum B12 or HTC testing for complete interpretation, Dr. Wilson said.
If faced with discordant vitamin B12 laboratory results in a patient with strong clinical features of deficiency, “just tell the clinicians to treat,” she advises, to avoid neurological impairment. “Vitamin B12 deficiency is easily corrected.”
Our next case is a case of anemia,” said Dr. Chabot-Richards as she described test results from a 12-year-old African-American girl who presented to the emergency department with acute hip pain. A CT scan and CT angiogram revealed a bony infarction in the femoral neck, and the girl’s CBC and peripheral blood smear showed evidence of anemia. Her blood smear was clearly abnormal, with numerous classic sickle cells with pointed ends, as well as other elongated red blood cells with blunt ends. Howell-Jolly bodies and target cells were present. The patient also had an elevated reticulocyte count at 3.7 percent.“This was sent out for hemoglobin electrophoresis. Alkaline electrophoresis is the classic screening test we do to evaluate for abnormal hemoglobins,” Dr. Chabot-Richards said. “It often reflexes to acid depending on our results.”
The patient had 42 percent abnormal hemoglobin running in the S/D/G lane and 46 percent running in the C/E/O/A2 lane. Hemoglobin F was two percent. A Sickledex test was positive, and the patient was diagnosed with sickle cell/hemoglobin C disease.
“This is a patient who has co-inheritance of two different β-chain variants: hemoglobin S and hemoglobin C,” Dr. Chabot-Richards said. Both are common variants in the African population, so it’s possible to see them combined.
The girl had no normal β-chain and was unable to make normal hemoglobin A. The combination of sickle cell and hemoglobin C causes a sickling disorder similar to sickle cell anemia. These patients typically have a slightly milder clinical picture and longer red blood cell survival, which leads to having a higher hemoglobin. Patients also have chronic hemolysis and are at risk for femoral neck necrosis and bone marrow infarction. Compared with patients with sickle cell anemia, patients with sickle cell hemoglobin C disease usually have delayed hyposplenism.
“These patients don’t typically have true sickle cells or true hemoglobin C crystals,” Dr. Chabot-Richards said of the blood smear findings. “Instead, you see more unusual, elongated cells with rounded ends.” Clam- or boat-shaped cells are increased, as are target cells.
In comparison, patients with sickle cell trait have one normal β-chain and one sickle β-chain. Electrophoresis would show 35 to 45 percent hemoglobin S, with the remainder normal hemoglobin A. The majority of sickle cell trait patients are asymptomatic with cells that rarely sickle, and have normal CBC parameters and normal peripheral blood cell appearance. “They do have an increased incidence of renal medullary carcinoma, a risk they share with people who have sickle cell anemia,” she said.
Sickle cell anemia patients are homozygous for the S β-chain, and electrophoresis results show greater than 80 percent hemoglobin S. These patients have chronic hemolytic anemia, and their cells sickle in low-oxygen environments. “These patients are typically hyposplenic, so their spleens infarct at a fairly young age,” Dr. Chabot-Richards said. Sickle cell anemia patients are also at risk for death from infection, stroke, or respiratory failure due to lung infarction.
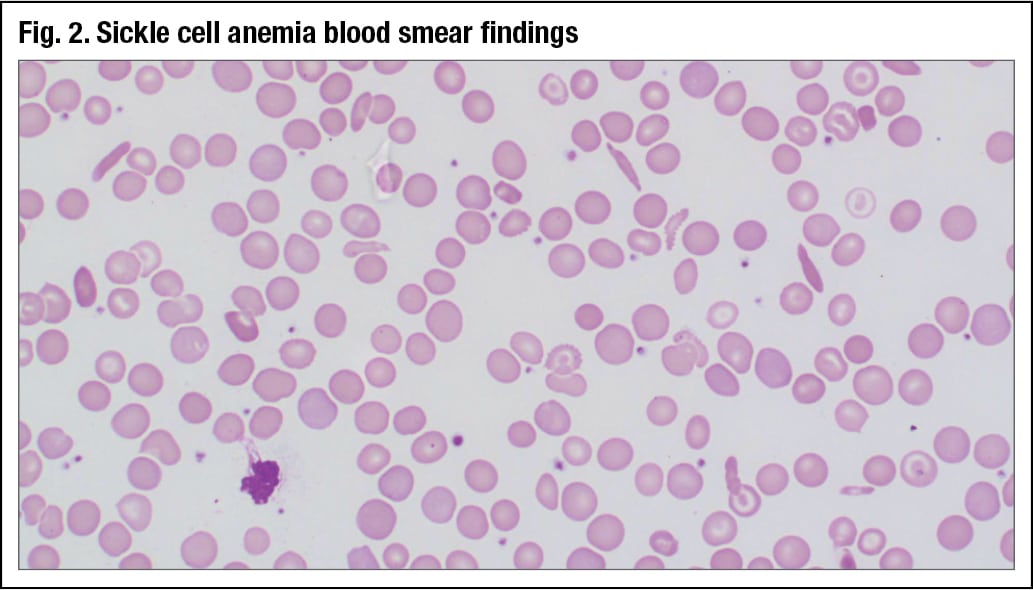
Peripheral blood smear findings of a sickle cell anemia patient show numerous elongated cells, much more pointed on the ends than what was seen in the 12-year-old girl (Fig. 2). “There is a lot of anisopoikilocytosis, so the cells have a higher red cell distribution width,” Dr. Chabot-Richards said.
 In a comparison of findings of sickle cell anemia patients and sickle C disease patients, she pointed out that while both patients have anemia, it is typically more severe in those with sickle cell anemia. Sickle cell anemia patients also typically have higher reticulocyte counts, higher WBC counts owing to the inflammatory process, higher bilirubin, and higher LDH because of increased hemolysis.
In a comparison of findings of sickle cell anemia patients and sickle C disease patients, she pointed out that while both patients have anemia, it is typically more severe in those with sickle cell anemia. Sickle cell anemia patients also typically have higher reticulocyte counts, higher WBC counts owing to the inflammatory process, higher bilirubin, and higher LDH because of increased hemolysis.
Sickle cell trait is fairly common and can be seen in combination with other hemoglobinopathies, which have different clinical manifestations, such as sickle cell/hemoglobin D. These patients have milder disease and usually a stable hemoglobin level. Sickle cell/hemoglobin O-Arab is a severe sickle disease; patients have one O-Arab β-chain and one sickle β-chain. “Usually, the hemoglobin S is at a slightly higher percentage than the O because the O is such a deleterious mutation,” Dr. Chabot-Richards said.
Another variation is sickle cell C-Harlem, which usually is present at a slightly higher percentage than the S on electrophoresis.
“If you have sickle cell trait together with an α-chain variant, it’s usually clinically silent,” Dr. Chabot-Richards said.
It is not uncommon for patients to have sickle hemoglobin and α-thalassemia co-inherited. These patients have decreased hemolysis and soft tissue damage, so the combination is slightly protective for them, though they have increased bone infarction and osteonecrosis. Overall survival rates are not very different.
If the sickle cell anemia is heterozygous and diagnosed along with an α-chain variant, typically the electrophoresis will show a decreased percentage of hemoglobin S.
Sickle hemoglobin with β-thal-assemia is also not uncommon. Patients who have a sickle cell β-chain with severe β-thalassemia leading to complete loss of protein function are unable to make normal hemoglobin A. They have less hemolysis than sickle cell anemia patients, and their hemoglobin parameters are typically higher. A peripheral blood smear from a patient with β-thalassemia shows a scattering of nucleated red blood cells at low power; sickle cells with pointed ends and numerous target cells are apparent at a higher power.
“Similar to B12 deficiency, sometimes there can be confusion with microangiopathic hemolytic anemia,” especially if the patient has very high anisopoikilocytosis, Dr. Chabot-Richards said. With insufficient experience, “it can be hard to distinguish between a sickle cell and a schistocyte.”
Sickle cells typically are larger and more uniform in size than schistocytes. The clinical presentations of sickle cell anemia and microangiopathic hemolytic anemia should be distinct.
Hemolysis is associated with thrombocytopenia, increased bilirubin and LDH, and decreased haptoglobin, so it is helpful to use ancillary tests to help determine the degree of hemolysis.
“Inheritance of any sickle cell hemoglobin will cause a positive Sickledex, so it’s important to realize that a positive Sickledex does not necessarily equal sickle cell disease in the patient, or even that the patient will sickle clinically,” Dr. Chabot-Richards said.
A 44-year-old woman returned to her doctor six months after a routine CBC that showed increased platelets. The blood smear at follow-up showed increased platelets with a range in size but normal granulation. The woman had no history of thrombotic or hemorrhagic events and no medication history, and she had normal coagulation and iron studies, LDH, and liver function tests.The patient’s hematologist recommended a bone marrow biopsy because of the sustained thrombocytosis. The patient wanted to avoid a biopsy and requested additional pre-procedure workup.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
