Sherrie Rice
April 2023—Molecular testing guidelines say that the neoplastic cell content of each specimen should be assessed, but there are no formal recommendations or guidelines on how to do the assessment.
Last year, current and past members of the CAP Molecular Oncology Committee set forth 13 recommendations, beginning with the definition of neoplastic cellularity and ending with quality assurance (Devereaux KA, et al. Arch Pathol Lab Med. 2022;146[9]:1062–1071). In an AACC session, Joel Moncur, MD, PhD, MS, a member of the committee and director of the Joint Pathology Center, Silver Spring, Md., explained why.
“Assessing neoplastic cellularity is an imprecise process, and we knew that because of a study done by the Molecular Oncology Committee” and reported in 2013, he said (Viray H, et al. Arch Pathol Lab Med. 2013;137[11]:1545–1549).
It was a prospective, multi-institutional diagnostic trial that found “there was low interobserver precision among pathologists and laboratories for the assessment of the percentage of neoplastic cells. In fact, in over half of the cases that were part of that study,” he said, “more than 10 percent of the participants overestimated neoplastic cellularity, which puts them at risk of a false-negative result.”
Insurers will often reimburse for mutation profiling only one time, Dr. Moncur said. “So if a test is conducted on an insufficient sample and the mutation is missed, then that patient may never again be offered the opportunity to be tested, and thus could miss the opportunity for a life-extending or lifesaving therapy.”
More recently, in 2019, the committee designed a questionnaire and performance challenge to help explain why neoplastic cellularity assessments can be imprecise. “And we discovered amazing things,” Dr. Moncur said.
They found that laboratories are using different methods to assess neoplastic cellularity, and CAP proficiency testing data makes clear that different methods produce different results. Laboratories also are using different definitions of neoplastic cellularity. Seventy percent of the 57 laboratories from which data were derived appropriately use the cell number method: the number of neoplastic cells compared to the overall number of cells in the tissue that will be tested. But 28 percent of laboratories were using the cell area method: the area of neoplastic cells compared to the overall area of the tissue. Defining neoplastic cellularity by cells or nuclei is considered to be more accurate because the number of cells or nuclei correlates directly with DNA content.
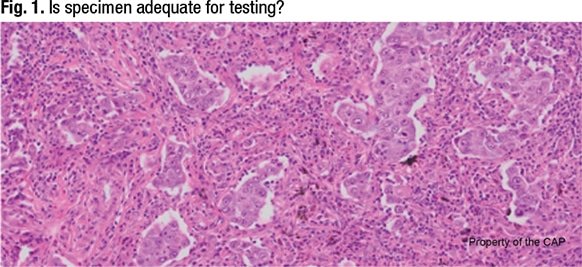
In Fig. 1 is an image of a lung cancer. If the laboratory’s molecular assay requires samples with at least 20 percent neoplastic cellularity, Dr. Moncur asked, is this specimen adequate for testing? The answer, he said, is no.
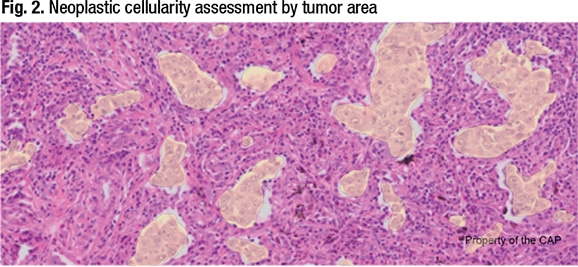
If the tumor cells in the Fig. 1 image of lung carcinoma are highlighted (Fig. 2) and the area method is used, “we could easily get to 20 percent neoplastic cellularity and call this specimen adequate. But that’s not the right way to do it,” Dr. Moncur explained, “because the DNA content of tumor is based on cell number as opposed to cell area. So this is how we determine the criterion standard, or the gold standard, for determining neoplastic cellularity, which is the number of neoplastic cells compared to the number of benign cells.”
In Fig. 3 is a demonstration of how the Molecular Oncology Committee develops the criterion, or gold, standard for each image. A red dot is placed over every neoplastic cell in the lung carcinoma image and a green dot over every benign cell in the background—292 red dots and 2,855 green dots, which is a 9.3 percent neoplastic cellularity. “So it’s important for laboratories to rely on cell number, which may be difficult when you have very large neoplastic cells and very small lymphocytes and other benign cells in the background, but that’s the best method to use to assess neoplastic cellularity,” he said.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
