Charna Albert
April 2023—With serous ovarian carcinomas and other gynecologic tumors, it’s molecular testing that increasingly sets the treatment course. “As pathologists, it’s exciting to us,” said Sadia Sayeed, MD, speaking at CAP22. “We’re learning that the immunostains we’re interpreting have greater implications than we ever thought they could.”
In a session on ancillary testing in gynecologic pathology, Dr. Sayeed, assistant professor of pathology and director of cytopathology, Virginia Commonwealth University Health, and Leslie M. Randall, MD, MAS, Dianne Harris Wright professor at VCU and division director of gynecologic oncology, VCU Health, reviewed the molecular classification of ovarian and cervical cancer and used their cases to highlight evolving practices in molecular diagnostics and treatment. One example: Treatments for low-grade and high-grade serous ovarian cancer are diverging, and next-generation sequencing can determine the grade when the pathology is equivocal.
Second of two parts. Last month:
Spotlight on ancillary tests in endometrial cancer
“Not only will NGS give you your definitive p53,” Dr. Randall said, “it will give you other markers that will increase your confidence in the low grade versus high grade.”
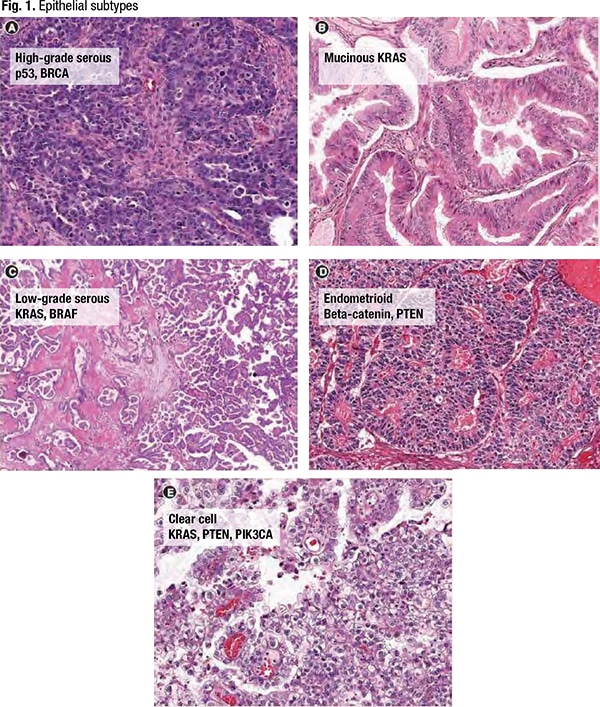
Of the five common epithelial subtypes of ovarian cancer seen in Fig. 1, high-grade serous is by far the most prevalent. High-grade serous tumors are characterized by the p53 mutation most commonly and often are associated with the BRCA mutation, though “it doesn’t necessarily have to be BRCA,” she said. “It just has to be in that pathway.” Mucinous tumors are driven by KRAS, “just like colorectal tumors. And we now treat them like colon cancer, with colon cancer chemotherapy regimens.” Low-grade serous tumors are characterized by KRAS and BRAF. The endometrioid subtype, like endometrial cancer, is driven by beta-catenin and PTEN mutations. And clear cell ovarian cancer is commonly associated with PTEN and KRAS, though “PIK3CA-ARID1A is coming online as a common somatic gene for clear cell tumors.”
When The Cancer Genome Atlas characterized high-grade serous ovarian cancer, Dr. Randall said, the strong degree of genomic instability was a surprise. “It’s true chromosomal disarray in high-grade serous ovarian cancer, and it probably goes back to it being associated with the p53 mutation,” she said. “That’s what makes it more chemosensitive and more sensitive to other therapies like PARP inhibitors. We also learned from the TCGA that BRCA1 was often mutated in these tumors, and at the time we didn’t realize it, but this was picking up both germline and somatic mutations.” BRCA2 also was present, as was CSMD3, NF1, CDK12, FAT3, GABRA6, and RB1 (TCGA Research Network. Nature. 2011;474[7353]:609–615). “But p53 was by far the most common genetic aberration in ovarian cancer.”
The TCGA characterized cervical cancer in 2017. Until that point, “we thought cervical cancer was all about HPV,” Dr. Randall said. “HPV E6 and E7 integrate into the host genome and cause malignant transformation of a precursor CIN lesion. And then breakdowns of the immune signaling and the basement membrane cause that to become an invasive tumor. All this is important because immunotherapy is an important treatment for cervical cancer and it’s based on this biology.”
The TCGA, however, found that “a lot of tumors had PIK3CA mutations, and a lot had HER2 overexpression.” That information was used to show that therapies targeted to those mutations have some effect in cervical cancer. “They don’t have a big effect,” she said. “And it’s hard to do cervical cancer studies in the U.S. because it’s not as common as endometrial or ovarian cancer. So we’re early in our study of targeted therapies for these different mutations in cervical cancer.” Patients with new cervical cancer diagnoses aren’t tested routinely for PIK3CA or HER2, “but when we have patients with recurrent disease, we will often send off tumor testing for NGS to look for these mutations to create treatment options.” As of now, she noted, the only biomarker testing required in first recurrence or a stage four diagnosis is PD-L1.
At VCU, Dr. Sayeed said, the only PD-L1 testing performed in-house is on lung cancer samples. “That’s what we validated our clone for—22C3 is the clone we have in-house. Anything that hasn’t been validated at our institution we’re sending out for interpretation.” If the clone hasn’t been validated for the specific tissue being tested, she said, “then you should not be interpreting PD-L1.”
For cervical cancer, Dr. Randall said, only the 22C3 antibody is approved as a companion diagnostic to pembrolizumab. And the combined positive score (CPS)—which tests tumor cells and immune cells for PD-L1 expression—should be taken, rather than the tumor proportion score (TPS). Cemiplimab, an immunotherapy approved in Canada and Brazil, is PD-L1 agnostic. “The cemiplimab trials used a tumor proportion score and a different clone and got different results,” she said. “So the PD-L1 expression didn’t matter in that study, but we think that’s possibly because of the way it was measured.”
When a physician requests PD-L1 testing at VCU, Dr. Sayeed said, “we ask them to specify which clone they want. And depending on the site we’ll decide whether it requires a CPS or a TPS,” which is usually decided by the site of the primary tumor. “So if it’s a cervical primary, they’ll know they have to use a CPS.” For lung primaries, they’re using only the TPS. “So we ask the providers which clone we should be ordering because they’re going to have to make the decision of how they’re treating it—is it a cervical primary that’s metastasized to the lung, or is it truly a lung primary.”
In designing the immunotherapy trials, Dr. Randall said, “at first we didn’t know that PD-L1 was going to be helpful, predictive or otherwise. So some of the early trials didn’t build in PD-L1, or we knew it was important but didn’t know how to measure it.” In some studies they enriched the population for squamous cell carcinoma. “We thought the expression was more common in squamous and in adenocarcinoma. But what we learned from doing the trials is that it’s not necessarily the case. You just have to measure the PD-L1. That’s what’s been truly predictive of response.”
 In the trials, they found that 90 percent of cervical cancers—squamous, adenocarcinoma, or adenosquamous-type—are PD-L1 positive. “When we first looked at PD-L1, it was in the recurrent population, and that’s enriched for PD-L1 negative. So at first we thought 50-50,” she said. The frontline trials revealed the true percentage. “We were shocked,” she said. “Nearly all of the common-type cervical cancers are PD-L1 positive.”
In the trials, they found that 90 percent of cervical cancers—squamous, adenocarcinoma, or adenosquamous-type—are PD-L1 positive. “When we first looked at PD-L1, it was in the recurrent population, and that’s enriched for PD-L1 negative. So at first we thought 50-50,” she said. The frontline trials revealed the true percentage. “We were shocked,” she said. “Nearly all of the common-type cervical cancers are PD-L1 positive.”
“We’re building the plane at 30,000 feet,” she continued. “We’re doing the trials and learning the biomarkers as we do the trials, and when we get to the end of the trial and look back, we think, ‘if we’d known about the biomarker we may have designed it differently.’ And sometimes we do it over and sometimes it’s not worth doing over.”
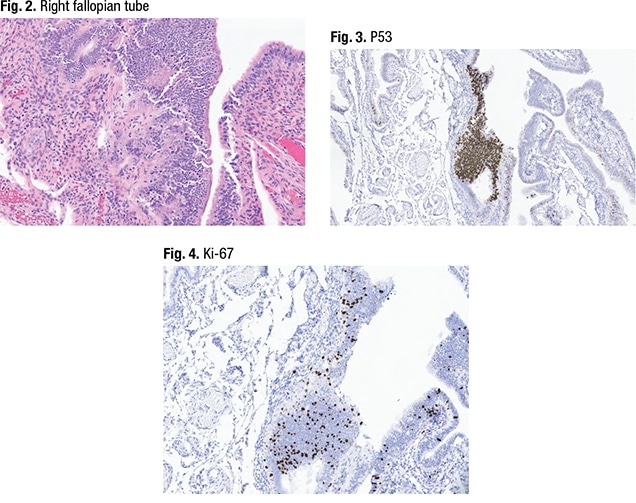
Dr. Sayeed shared the case of a woman in her mid-40s who has a BRCA mutation and who had a prophylactic salpingo-oophorectomy. In Fig. 2 is a section of the right fallopian tube. “Histology-wise, on the righthand side, this is a pretty normal looking fallopian tube—sort of pseudostratified,” she said, with some ciliated epithelium. The lefthand side seems to have a tangential section of normal epithelium that looks more cellular, she said, noting the cilia on the surface. “What we do at VCU is perform p53 and Ki-67 up front on all fimbriated ends of the fallopian tube on these prophylactic specimens. And when we did that we had this interesting finding.”In the right fallopian tube, the p53 testing (Fig. 3) revealed p53 wild-type on the right side of the image, “where it looks normal.” P53 overexpression was also seen (middle, in brown). “When we did the Ki-67 (Fig. 4) we also noticed an increase in Ki-67.” But morphologically, she said, “it doesn’t look like carcinoma. In fact it looks tangential compared to the rest of the fallopian tube.”
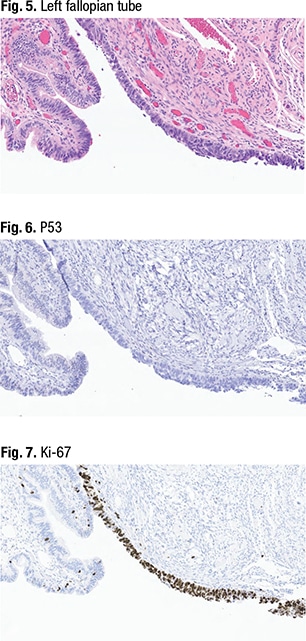
In the other fallopian tube (Fig. 5), “we noticed something a little more dramatic,” she said. On one section of the fimbriated end (right, in purple), “we had some tumor cells. And these tumor cells have a high nucleus-to-cytoplasmic ratio—you’ve lost the ciliated epithelium of a normal fallopian tube.” Mitotic activity also could be seen. “So morphologically this looks like carcinoma,” she said, limited to the surface of the fimbriated end. “We thought this looked like a serous tubal intraepithelial carcinoma lesion,” or STIC.
P53 immunostaining (Fig. 6) revealed a total loss of p53 in the area with the carcinoma-like morphologic appearance. “You can see a control in the background,” at far left, “which is a wild-type expression of a normal-looking fallopian tube,” she said. Similarly, Ki-67 (Fig. 7) was highly elevated in the area that looked like a STIC (in brown) “but sporadic in the normal-looking tubal epithelium,” at far left. They called the right fallopian tube a serous tubal intraepithelial lesion (STIL), and the left fallopian tube a STIC.
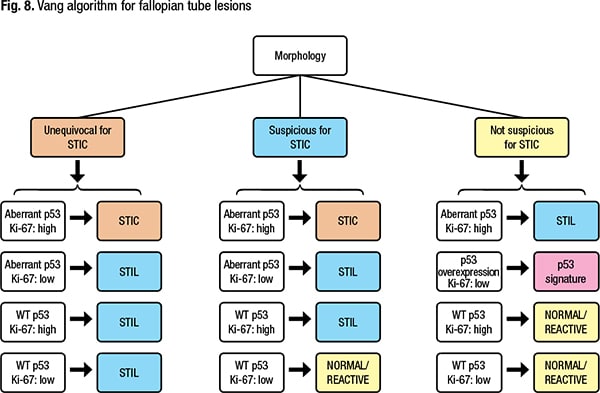
In diagnosing a fallopian tube lesion, Dr. Sayeed said, both morphology and immunostaining should be taken into consideration. Many follow the Vang algorithm for fallopian tube lesions (Fig. 8) (Vang R, et al. Histopathology. 2022;81[5]:542–555). “This algorithm is nice because it separates what your impression is morphologically and then [shows] how to use your immunostains to support that diagnosis,” she said. According to the Vang algorithm, if the morphology is unequivocal for STIC, p53 is aberrant, and Ki-67 is high, “you can call it a STIC.” If under similar circumstances the Ki-67 is low—a cutoff of 10 percent is used—it’s a STIL. And if p53 is wild-type, the lesion would be considered a STIL whether or not the Ki-67 is elevated, she said. “So if you’re suspicious for STIC but it’s not unequivocal morphologically,” aberrant p53 and elevated Ki-67 can help confirm that the lesion should be in the STIC category.
If the specimen isn’t morphologically suspicious for STIC “but you did the immunostains anyway,” she said, “you may find that you have a STIL lesion,” as seen in the case example, which had normal morphology but p53 overexpression and elevated Ki-67. P53 overexpression combined with low Ki-67 is known as a p53 signature. “This is controversial,” she said, noting that Vang, et al., say it shouldn’t be reported clinically. “I do report it if I have it. I don’t know if it’s useful information for the clinical side, but it is sometimes helpful for the patient who got prophylactic surgery, to know that there was some finding and a reason they had that surgery in the first place.” All other combinations, she noted, would be normal/reactive.
The clinical implications of STIC still are unclear, Dr. Sayeed said. One meta-analysis sought to determine if STIC is implicated in patients who develop peritoneal carcinomatosis following risk-reducing salpingo-oophorectomy (Steenbeek MP, et al. J Clin Oncol. 2022;40[17]:1879–1891). Steenbeek, et al., found that patients with isolated STIC at the time of risk-reducing surgery were at increased risk for developing peritoneal carcinomatosis, with the five- and 10-year risks 10.5 percent and 27.5 percent, respectively, versus 0.3 percent and 0.9 percent in patients without STIC. “But the questions that still remain are what criteria did they use for STIC, how rigidly did they apply those criteria, and did they exclude high-grade serous carcinoma if those were found subsequently—we don’t know the answer to that question.” Larger studies are needed to determine the clinical implications of STIC and if the results from the meta-analysis are reproducible, she said.
At VCU, prophylactic bilateral salpingo-oophorectomy specimens are grossed using the SEE-FIM (sectioning and extensively examining the FIMbriated end) protocol. And to ensure all tubal lesions are found, p53 staining is performed up front on all fimbriated ends of the fallopian tube. “We’re also using the p53 and Ki-67 combination as suggested by the Vang algorithm,” Dr. Sayeed said. “If you see a STIC lesion in the setting of invasive high-grade serous ovarian carcinoma, do you need to do a p53 or Ki-67? Probably not. But in the context of the prophylactic salpingo-oophorectomy, it is a nice way to confirm the diagnosis.”
STIL can be diagnosed descriptively as “epithelial atypia,” she noted, “if you’re not sure if it qualifies as a STIL lesion,” and a comment in the pathology report can explain that the lesion does not meet diagnostic criteria for STIC. “Clinically, I think that’s the most important thing.”
Said Dr. Randall: “You should be doing the SEE-FIM protocol at a minimum on all patients who are BRCA-mutation carriers, because they could have cancer. If they have cancer we need to find it, and we need to stage it in most cases.”
Though the STIC lesion is considered a precursor to tubal cancer, “we don’t really know what to do with STIC lesions when we find them,” Dr. Randall said. “We don’t go back and stage them. We started doing that and it was extremely low yield—they rarely have metastatic disease. What we do typically is monitor them closely with CA 125 exams and scans where indicated.” Though patients with STIC lesions aren’t given chemotherapy, “it’s important to find them. If you don’t find them, they [the patients] could have an occult cancer later on, and we may not be picking that up.” Many of these patients are under surveillance because of their BRCA status, she noted. “But not all of them are. And you’ll find these in patients who don’t have BRCA.”
On the pathology side, Dr. Sayeed said, “we look into the clinical history to see why the salpingo-oophorectomy was done. So even if we don’t see that it’s BRCA mutated, maybe there’s a family history of ovarian cancer. And that’s enough for us to go ahead and trigger that type of grossing.” And testing the fimbriated ends usually can be done in one or two slides. “It’s not a huge expenditure of energy on our end to find those cases.”
“If there is somebody with incidental STIC, we’ll find it,” she said.
A prospective clinical trial evaluating whether bilateral salpingectomy with delayed oophorectomy can reduce the risk of ovarian cancer to the same degree as full bilateral salpingo-oophorectomy in 35-year-old patients who have a BRCA mutation will measure the longitudinal outcomes of STIC, Dr. Randall said, as well as cancer diagnoses and other outcomes (NRG Oncology, NRG-CC008). “But learning that most of these cancers occur in the fallopian tubes gave us the option to do only a salpingectomy at 35 and wait until menopause to take out the ovaries.”
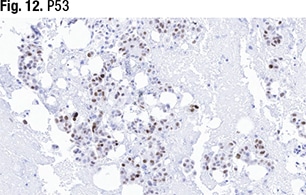
 Some cells stained weakly for p53 expression (Fig. 12). “We definitely don’t have any cytoplasmic staining. This is on the equivocal side—not exactly 80 percent but around that range. In these cases, where morphology isn’t 100 percent and neither is the p53, we would do NGS testing.” The case was verified as an ovarian serous carcinoma metastatic to the omentum. “We sent it for next-generation sequencing to look at the TP53 mutation and it came back as no TP53 mutation. So that takes us back into the low-grade serous carcinoma category.” Typically, she said, they issue an addendum to the report once they receive the NGS results.
Some cells stained weakly for p53 expression (Fig. 12). “We definitely don’t have any cytoplasmic staining. This is on the equivocal side—not exactly 80 percent but around that range. In these cases, where morphology isn’t 100 percent and neither is the p53, we would do NGS testing.” The case was verified as an ovarian serous carcinoma metastatic to the omentum. “We sent it for next-generation sequencing to look at the TP53 mutation and it came back as no TP53 mutation. So that takes us back into the low-grade serous carcinoma category.” Typically, she said, they issue an addendum to the report once they receive the NGS results.
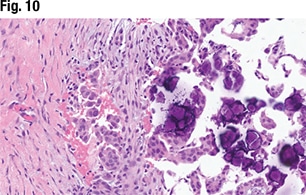 Low-grade and high-grade serous carcinomas have distinct and clearly defined histologic criteria, Dr. Sayeed said. High-grade serous carcinomas have marked cytologic atypia (large and pleomorphic with greater than 3:1 in size variation), whereas low grade have more mild to moderate cytologic atypia. And high-grade serous carcinomas should show an increase in mitoses, with greater than 12 mitoses in 10 high-power fields. “That might be difficult in an omental biopsy, where you might not have 10 high-power fields.” The same may be true for cytology specimens, she noted. “Architecturally high grades can be solid—sometimes they can be granular and papillary as well, whereas we tend to think of the low grades as having more papillary architecture.” And sometimes “it does come down to the p53 mutation. You should see an aberrant p53 mutation on the high-grade serous side, but not so much on the low-grade serous.”
Low-grade and high-grade serous carcinomas have distinct and clearly defined histologic criteria, Dr. Sayeed said. High-grade serous carcinomas have marked cytologic atypia (large and pleomorphic with greater than 3:1 in size variation), whereas low grade have more mild to moderate cytologic atypia. And high-grade serous carcinomas should show an increase in mitoses, with greater than 12 mitoses in 10 high-power fields. “That might be difficult in an omental biopsy, where you might not have 10 high-power fields.” The same may be true for cytology specimens, she noted. “Architecturally high grades can be solid—sometimes they can be granular and papillary as well, whereas we tend to think of the low grades as having more papillary architecture.” And sometimes “it does come down to the p53 mutation. You should see an aberrant p53 mutation on the high-grade serous side, but not so much on the low-grade serous.”
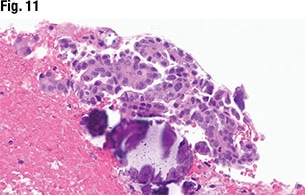 “If you do run into that equivocal problem,” she continued, “or you’re not quite sure it meets all the morphology criteria—maybe it’s a sampling issue—or the p53 stain isn’t working so well for you, I would recommend doing sequencing if at all possible.”
“If you do run into that equivocal problem,” she continued, “or you’re not quite sure it meets all the morphology criteria—maybe it’s a sampling issue—or the p53 stain isn’t working so well for you, I would recommend doing sequencing if at all possible.”
A low-grade serous tumor can occasionally become high grade; when it does it should have a p53 mutation. (Perhaps they were high grades all along, in Dr. Randall’s view, and there was merely diagnostic uncertainty.) Though there are reports of high-grade serous carcinomas with p53 wild-type expression (Chui MH, et al. Mod Pathol. 2021;34[2]:490–501), they are rare, Dr. Sayeed said. If in doubt, she suggests molecular testing. When she signs out the case in such situations, she lets the clinician know what the differential is and that TP53 status is being used to confirm the diagnosis.
 NGS, Dr. Randall said, often is needed anyway for BRCA and homologous recombinant deficiency testing. And NGS will find other markers associated with low- and high-grade serous carcinomas that can help confirm the diagnosis. “For low grade it’s KRAS and BRAF, and for high grade it’s BRCA and any of the other HR deficiency genes. So you can get additional information that supports what you think it is based on the p53, and it matters because the treatment paradigms are diverging,” she said. KRAS-driven therapies and MEC inhibitors are being developed for low-grade serous carcinomas, “and there’s the whole chemotherapy paradigm for high-grade serous ovarian. So it matters.”
NGS, Dr. Randall said, often is needed anyway for BRCA and homologous recombinant deficiency testing. And NGS will find other markers associated with low- and high-grade serous carcinomas that can help confirm the diagnosis. “For low grade it’s KRAS and BRAF, and for high grade it’s BRCA and any of the other HR deficiency genes. So you can get additional information that supports what you think it is based on the p53, and it matters because the treatment paradigms are diverging,” she said. KRAS-driven therapies and MEC inhibitors are being developed for low-grade serous carcinomas, “and there’s the whole chemotherapy paradigm for high-grade serous ovarian. So it matters.”
Though uterine sarcomas do have underlying biologic biomarkers, she said, “there’s no central biomarker that defines or drives these.”
Charna Albert is CAP TODAY associate contributing editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
