CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from the University of Texas Southwestern Medical Center. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Jing Xu, MD, PhD; Guanglu Shi, PhD
Ozlem Kulak, MD, PhD; Weina Chen, MD, PhD
Prasad Koduru, PhD; Jeffrey Gagan, MD, PhD
March 2021—Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is one of the most common lymphoproliferative diseases. It is a CD5-positive B-cell neoplasm of monomorphic small mature B cells. One of the characteristics of CLL/SLL is its heterogeneity, not only among individuals but also within individual patients.1 The cytogenetic and molecular variants are dynamic during disease progression and in response to targeted therapies. Here, we present a patient with CLL/SLL whose disease was characterized by molecular and cytogenetic evolution during various stages of disease progression, which culminated in diffuse large B-cell lymphoma (Richter syndrome).
Case. A 53-year-old male presented to our hospital in 2019 with progressive disease of CLL/SLL despite extensive treatment. The patient was first diagnosed with CLL/SLL at an outside hospital in 2004 by lymph node biopsy for his cervical lymphadenopathy. Flow cytometry was positive for CD38 and ZAP-70. No cytogenetic or molecular tests were done at that time. He then underwent treatment with fludarabine/rituximab with good response. His first instance of disease progression occurred in 2007, when he was treated with fludarabine/cyclophosphamide/rituximab, and again experienced a good response. Progressive lymphadenopathy was next observed in 2010. A bone marrow biopsy at that time showed extensive marrow involvement by CLL/SLL. Karyotype and a CLL/SLL FISH panel did not show any abnormalities. He was then treated with bendamustine/rituximab, which was still unable to control the disease. Another bone marrow biopsy in 2012 showed marrow involvement with 44 percent CLL/SLL cells. Karyotype analysis demonstrated a 13q deletion. He was then treated with a regimen of ibrutinib and rituximab for four years.
In 2016, he experienced progressive lymphadenopathy again. A next-generation sequencing assay was performed on his bone marrow sample at an outside hospital. It covered 29 genes for single nucleotide variants, as well as insertions and deletions (indels). It showed a mutation in BTK, which is associated with ibrutinib resistance, as well as mutations in the SF3B1 and XPO1 genes (Table 1). Two TP53 point mutations in cis were also detected, at lower allele frequencies than the other variants, although exact allele frequencies were not reported. Consequently, he was switched from ibrutinib to venetoclax/rituximab and later idelalisib/rituximab therapies. Neither of these therapies successfully controlled the disease.
In February 2019, a PET CT showed intra-abdominal adenopathy with marked hepatosplenomegaly. Image-guided biopsy of a retroperitoneal lesion showed prolymphocytic transformation of CLL/SLL with no evidence of large cell transformation. He then underwent CD19 CAR T-cell therapy in March 2019. A bone marrow biopsy after the CAR T-cell therapy was MRD negative by flow cytometric analysis. However, a PET scan done one month later showed hypermetabolic adenopathy; therefore, ibrutinib therapy was reinstituted.
A repeat bone marrow biopsy done at our hospital in November 2019 demonstrated infiltration by CD5(+)/CD10(–) diffuse large B-cell lymphoma, likely representing Richter syndrome (Fig. 1). Lymphoma cells were positive for MYC, BCL2, MUM-1, and TP53 by immunohistochemistry, but negative for BCL6. Karyotype of the bone marrow aspirate was abnormal with three related clones containing multiple structural abnormalities, including rearrangements leading to extra copies of 8q and 11q, and a 17p deletion (Fig. 2). Consistent with the karyotype result, the FISH study also showed evidence of extra copies of MYC (8q24) (in 92.5 to 96.5 percent of the cells examined) and ATM (11q22.3) (in 91.5 percent of the cells examined), and deletion of TP53 (17p13.1) (in 80 percent of the cells examined) (Fig. 3). Notably, the del(13q) abnormality seen in the 2012 sample was not detected in this bone marrow by either karyotype or FISH analysis. The immunoglobulin heavy chain variable (IGHV) gene was unmutated, which was correlated with Richter syndrome and poorer clinical outcomes.2,3
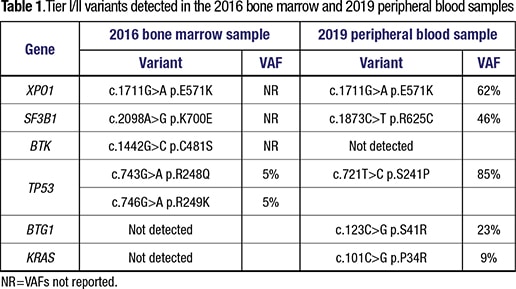 To further characterize the disease, we performed an NGS study on a peripheral blood sample using our institution’s 1,385-gene panel (full gene list at: https://j.mp/3oYr2bf). This assay reports single nucleotide variants, indel, copy number, and gene fusions. Mutations in multiple genes were detected, including TP53, XPO1, SF3B1, BTG1, and KRAS (Table 1). Interestingly, only the XPO1 mutation was consistent with the 2016 sample. However, the 2016 assay did not interrogate BTG1 or KRAS. Although TP53 and SF3B1 were mutated in both samples, the variants observed were completely different.
To further characterize the disease, we performed an NGS study on a peripheral blood sample using our institution’s 1,385-gene panel (full gene list at: https://j.mp/3oYr2bf). This assay reports single nucleotide variants, indel, copy number, and gene fusions. Mutations in multiple genes were detected, including TP53, XPO1, SF3B1, BTG1, and KRAS (Table 1). Interestingly, only the XPO1 mutation was consistent with the 2016 sample. However, the 2016 assay did not interrogate BTG1 or KRAS. Although TP53 and SF3B1 were mutated in both samples, the variants observed were completely different.
The patient further underwent clinical trial ARQ531 and then DA-R-EPOCH/ibrutinib treatments. Unfortunately, the disease continued to progress despite those treatments and the patient passed away due to pulmonary complications. No autopsy was performed.
 Discussion. CLL/SLL is characterized by the high degree of variability in its disease course. While some patients maintain an indolent disease course, others develop refractory disease to chemotherapies. In approximately five percent to 10 percent of patients, CLL/SLL can undergo transformation into an aggressive lymphoma, most commonly DLBCL.4 CLL/SLL progression is a complex and dynamic process involving the development of different subclones with changing dominance over time. It has been reported that driver mutations can be categorized in two groups: clonal mutations, which are present in all leukemic cells and represent early events in the tumorigenesis process; and subclonal mutations, which exist in a subset of tumor cells and represent late events.5 The number of subclonal mutations is usually increased with treatments.5
Discussion. CLL/SLL is characterized by the high degree of variability in its disease course. While some patients maintain an indolent disease course, others develop refractory disease to chemotherapies. In approximately five percent to 10 percent of patients, CLL/SLL can undergo transformation into an aggressive lymphoma, most commonly DLBCL.4 CLL/SLL progression is a complex and dynamic process involving the development of different subclones with changing dominance over time. It has been reported that driver mutations can be categorized in two groups: clonal mutations, which are present in all leukemic cells and represent early events in the tumorigenesis process; and subclonal mutations, which exist in a subset of tumor cells and represent late events.5 The number of subclonal mutations is usually increased with treatments.5
In this case, three cytogenetic tests were done at different time points of the disease (2010, 2012, 2019) with different results. While the first test was normal, the second test done in 2012 had a 13q deletion in the leukemia cells, and the third test done in 2019 showed complex karyotypes of three related clones with multiple structural abnormalities including extra copies of 8q and 11q, and a 17p deletion (Table 2). 13q deletion is one of the most frequent chromosomal abnormalities observed in CLL/SLL, and it is associated with a favorable prognosis if occurring as the sole genetic abnormality.6 Complex karyotype and 17p deletion are associated with Richter syndrome and adverse prognosis.3,6 The differences in these three cytogenetic results are consistent with the theory of clonal and subclonal evolution of the leukemia cells. In addition, the fact that the 13q deletion abnormality detected in the 2012 sample was not present in the 2019 sample raised the possibility that these were distinct subpopulations of the tumor cells as far back as 2012.
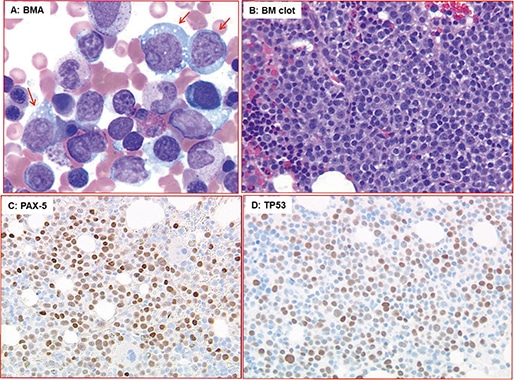
Fig. 1. Diffuse large B-cell lymphoma in bone marrow. A) Scattered large lymphoma cells (red arrow) with prominent nucleolus in bone marrow aspirate (BMA, Wright-Giemsa stain, ×500). B) Sheets of large lymphoma cells in BM clot section (H&E stain, ×500). C and D) Lymphoma cells express B-cell marker (PAX5) in C and TP53 in D (immunohistochemical stain, ×200).
The two NGS tests done in 2016 and 2019 also had distinctive results, which raised the question whether the DLBCL in fact represented a de novo neoplasm. The XPO1 p.E571K mutation was the only variant consistently detected in both tests. XPO1 (exportin) is a member of the importin-β superfamily of karyopherins that mediates the translocation of many proteins and RNAs to the cytoplasm, and thus regulates critical signaling pathways and cellular functions. E571K is a hotspot mutation that has been shown to alter XPO1 affinity for nuclear export signals.7 Notably, XPO1 mutations have been reported to be associated with Richter syndrome,8-10 but are rarely detected in de novo DLBCL,11 which supported the notion that the DLBCL arose from the prior CLL/SLL. The XPO1 E571K mutation likely represented the clonal mutation. Subclonal evolution and genetic divergence initiated later and resulted in cells with different mutations and chromosome abnormalities.
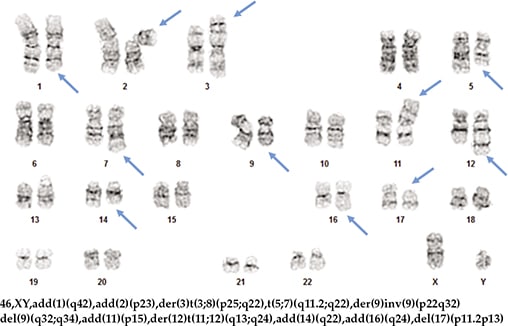
Fig. 2. Karyotype of one of the clones identified on the 2019 bone marrow sample.
Point mutations in SF3B1 and TP53 genes were detected by both NGS tests, albeit at distinctive codons, suggesting these might represent subclonal mutations from an ancestor who was only XPO1 mutated. Indeed, the SF3B1 and TP53 mutations have been reported to be more often subclonal and represent late events in CLL/SLL.5 Both TP53 and SF3B1 mutations are associated with an increased risk of Richter syndrome and unfavorable prognosis.12,13
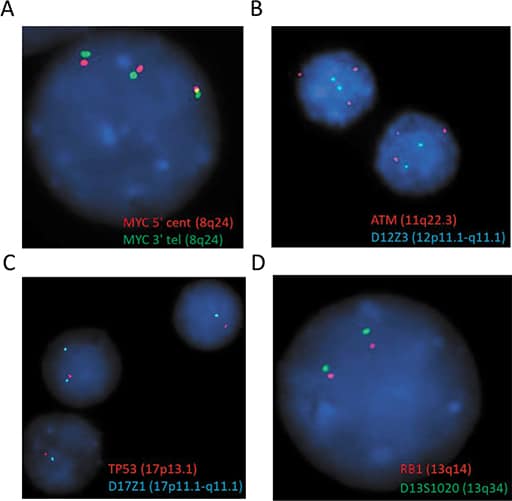
Fig. 3. FISH test showed extra copies of MYC (A), ATM (B) and deletion of TP53 (C). No evidence of deletion of RB1 was detected (D).
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
