CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Emory University School of Medicine. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Julia An, MD
Debra Saxe, PhD
Jaime Vengoechea, MD
Shiyong Li, MD, PhD
July 2023—Acute promyelocytic leukemia (APL) constitutes approximately five to eight percent of cases of acute myeloid leukemia (AML) and commonly presents in young adults.1 Prompt diagnosis is essential because of the frequent association with life-threatening disseminated intravascular coagulation (DIC) as well as the availability of therapeutic treatment options such as all-trans retinoic acid (ATRA) and arsenic trioxide (ATO). Two morphological variants of APL have been described: hypergranular (typical) and hypogranular (microgranular).
The hypergranular variant is associated with leukopenia and frequent abnormal promyelocytes with abundant cytoplasmic granules and bundles of Auer rods. Immunophenotypically, the abnormal promyelocytes demonstrate increased side scatter with low to absent expression of HLA-DR and CD34, as well as leukocyte integrins (CD11a, CD11b, and CD18). Variable CD13, uniform CD33, and strong cytoplasmic myeloperoxidase (MPO) expression are also characteristic features.1
On the other hand, the hypogranular variant is associated with leukocytosis and displays morphologic features of abnormal promyelocytes that have sparse or fine granulation and irregular bilobed or butterfly-shaped nuclei.1 Immunophenotypically, the abnormal promyelocytes demonstrate expression of CD13, CD33, and MPO with dim expression of HLA-DR and CD34 as well as aberrant expression of CD2 and/or CD56. CD2 expression has been associated with FLT3 mutations of which internal tandem duplications (ITD) make up the majority.2
GATA2 is located on chromosome 3q21.2 and encodes a zinc finger transcription factor, GATA2.3 GATA2 is critical in the formation and maintenance of hematopoietic stem cells as well as the production of megakaryocytes, mast cells, natural killer (NK) cells, and monocytes. De novo sporadic germline mutations or autosomal dominant inheritance of a mutated copy of GATA2 may lead to GATA2 deficiency.3 GATA2 deficiency is a complex multisystem disorder that may manifest with bone marrow failure, hematologic malignancies, and severe immunodeficiency.4 Pathogenic germline variants in GATA2 have been associated with a variety of clinical symptoms that have been classified into various groups such as MonoMAC (monocytopenia and mycobacterial infection), DCML deficiency (dendritic cell, monocyte, B and NK lymphoid), and Emberger syndrome.3 Here we describe a germline GATA2 variant in a patient with APL, which demonstrates an unusual morphology and immunophenotypic profile.
Case. A 21-year-old adopted male patient with a past medical history of autism spectrum disorder and obsessive-compulsive disorder presented to an urgent care center with nausea, vomiting, congestion, mild cough, and abdominal pain in August 2021. A complete blood count demonstrated hemoglobin of 11.7 g/dL (11.4–17.1 g/dL), white blood cell count of 12.4 × 103/µL (3.1–10 × 103/µL), and platelet count of 41.0 × 103/µL (126–362 × 103/µL). In addition, the WBC differential count revealed increased blasts (29 percent). Blood coagulation tests were significant for prothrombin time of 19.1 seconds (11.9–14.1 seconds) and INR of 1.66 (0.9–1.1). Repeat blood cultures demonstrated no growth, and testing for COVID-19, HIV, and hepatitis C viruses was negative. CT imaging of abdomen/pelvis revealed splenomegaly with findings suggestive of splenic infarct with consideration for lymphoma or other lymphoproliferative process. He was subsequently transferred to Emory University Hospital for further evaluation of suspected acute leukemia.
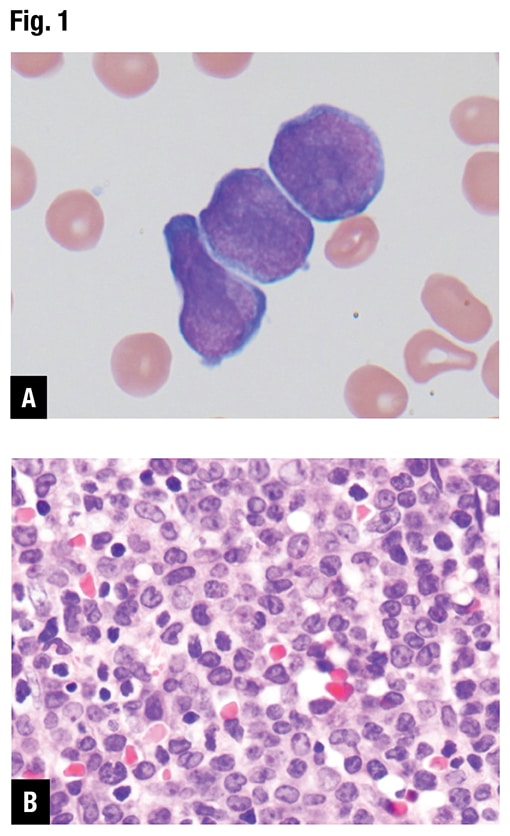
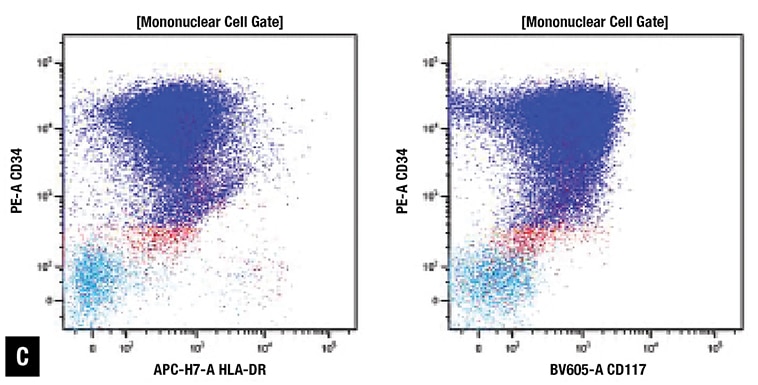
At Emory, peripheral blood smear review revealed approximately 70 percent blasts with scant basophilic cytoplasm and irregular nuclear contours (Fig. 1A). No definite Auer rods were identified, and neither classical abnormal promyelocytes nor their bilobed variants were evident. Bone marrow biopsy showed a hypercellular marrow with sheets of immature mononuclear cells with irregular nuclei and scant to moderate amount of cytoplasm (Fig. 1B). Flow cytometric immunophenotyping showed 86 percent of analyzed cells to express CD2, CD13, CD33, partial CD36, CD64, CD117, CD123, CD200, and CD45 (dim) with coexpression of bright CD34 and moderate HLA-DR. Representative flow cytometry dot plots are shown in Fig. 1C.
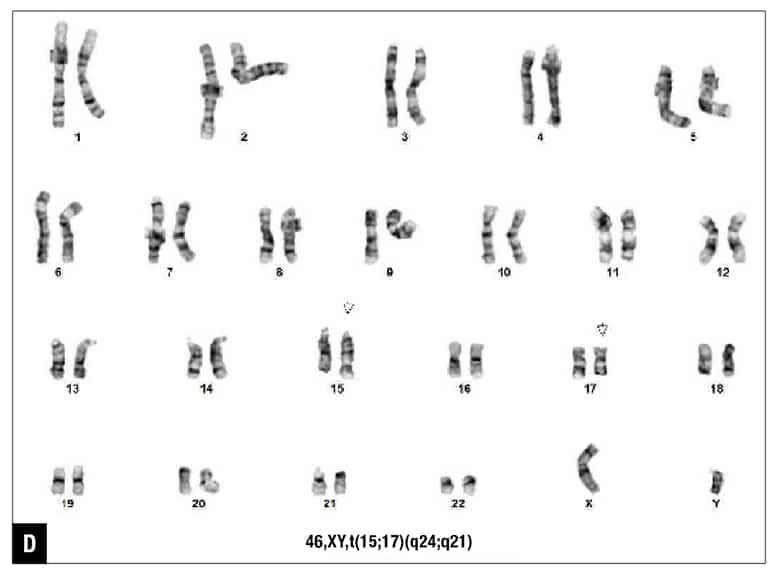
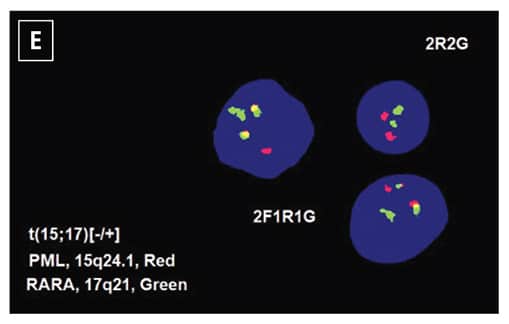
The patient was diagnosed with acute myeloid leukemia, and 7+3 chemotherapy regimen (cytarabine and daunorubicin) was initiated. Five days after the initiation of chemotherapy, routine chromosome analysis demonstrated an abnormal male karyotype with translocation between the long arms of chromosomes 15 and 17, t(15;17)(q24;q21), in 18 of 21 metaphases examined (Fig. 1D). Also, FISH confirmed the presence of t(15;17)(PML::RARA) in 82 percent of the interphase nuclei (Fig. 1E). Taken together with the morphologic and flow cytometry findings, the diagnosis was revised to APL following the results of ancillary studies,5 and ATRA therapy was initiated.
Next-generation sequencing was performed at Emory University Hospital using the in-house targeted myeloid mutation panel, which simultaneously detects single nucleotide changes, insertions and deletions, and internal tandem duplications of 75 genes involved in myeloid neoplasms (e.g. ABL1, FLT3, ASXL1, GATA2, etc.). Briefly, genomic DNA was isolated from bone marrow samples using a Qiagen DNA preparation kit. The targeted exons of the 75 genes were amplified using Anchored Multiplex PCR (AMP). Direct sequencing of the amplified enriched regions was performed on the Illumina NextSeq instrument, and the Emory Healthcare GenomOncology Pathology Workbench was used to analyze and annotate variants.
NGS analysis revealed GATA2 c.121C>G (p.P41A) in approximately 49 percent of alleles (Fig. 1F) and FLT3-ITD, 63-bp, with an allele frequency of 22 percent, resulting in 21 amino acid duplication (data not shown). One month after the initial diagnosis, a follow-up marrow sample demonstrated no definite morphologic evidence of APL. Flow cytometric immunophenotyping failed to identify any abnormal hematolymphoid cell populations. Routine chromosome analysis revealed a normal male karyotype. The patient was then initiated on consolidation therapy with ATRA and ATO and completed the therapeutic regimen in March 2022.
Bone marrow biopsy samples from April and July 2022 were hypocellular for age without evidence of residual disease. NGS performed on the April 2022 remission bone marrow demonstrated the disappearance of FLT3-ITD mutation, while the GATA2 c.121C>G (p.P41A) mutation is persistent at an allele frequency similar to that at diagnosis, suggestive of germline origin.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
