CAP TODAY and the Association for Molecular Pathology have teamed up to bring molecular case reports to CAP TODAY readers. AMP members write the reports using clinical cases from their own practices that show molecular testing’s important role in diagnosis, prognosis, and treatment. The following report comes from Aga Khan University in Karachi, Pakistan. If you would like to submit a case report, please send an email to the AMP at amp@amp.org. For more information about the AMP and all previously published case reports, visit www.amp.org.
Zeeshan Ansar, MBBS; Hareem Alam, MBBS
Muhammad Shariq, MBBS; Hasan Hayat, MBBS
Asghar Nasir, PhD; Tariq Moatter, PhD
March 2024—Case. An 87-year-old male with a clinical history of hypertension and sick sinus syndrome presented with a one-month history of fever, generalized weakness, and weight loss. There was no lymphadenopathy or hepatosplenomegaly on physical examination. Bone marrow examination was performed to evaluate for cytopenias. Complete blood counts showed hemoglobin of 9.0 g/dL, hematocrit 27.0 percent, WBC 1.92 × 109/L, ANC 0.23 × 109/L, and platelets 82 × 109/L. Peripheral blood film showed leukopenia with 21 percent blast cells. Also seen were anisopoikilocytosis, macrocytosis, and polychromasia of red blood cells, and thrombocytopenia.
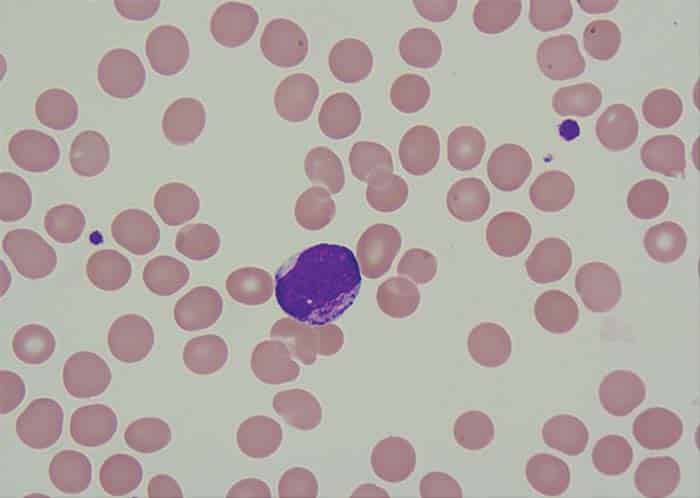
Fig. 1. Myeloblast exhibiting intracytoplasmic granules
Bone marrow aspirate showed 24 percent blast cells in total nucleated nonerythroid cell population. Blasts were medium to large with moderately abundant cytoplasm containing pink granules, open nuclear chromatin, and prominent nucleoli (Fig. 1). Normal hematopoiesis was seen in the background. Bone trephine was hypercellular and showed interstitial infiltration with blast cells. Immunophenotyping of blast cells by flow cytometry showed reactivity to CD13, CD33, cytoplasmic MPO, HLA-DR, CD117, and CD34. Bone marrow cytogenetic analysis was reported as 52~58, XY, add(6)(q27)[16]/46,XY[04]. The following chromosomes were gained: 1, 2, 6, 8, 9, 11, 13, 16, 18, 20, and 22 (Fig. 2). FISH assays for 5q deletion and trisomy 8 were positive (Figs. 3a and 3b). NPM1, FLT3-ITD, and D835 mutations by conventional PCR were not detected. The case was diagnosed as acute myeloid leukemia. The patient has thus far received one cycle of azacitidine chemotherapy; venetoclax was held after the first dose because of abnormal serum creatinine levels.
![Fig. 2. Bone marrow karyotype: 52~58, XY, add(6)(q27)[16]/46,XY[04]](https://www.captodayonline.com/wordpress/wp-content/uploads/2024/03/amp-ansar-fig2.jpg)
Fig. 2. Bone marrow karyotype: 52~58, XY, add(6)(q27)[16]/46,XY[04]
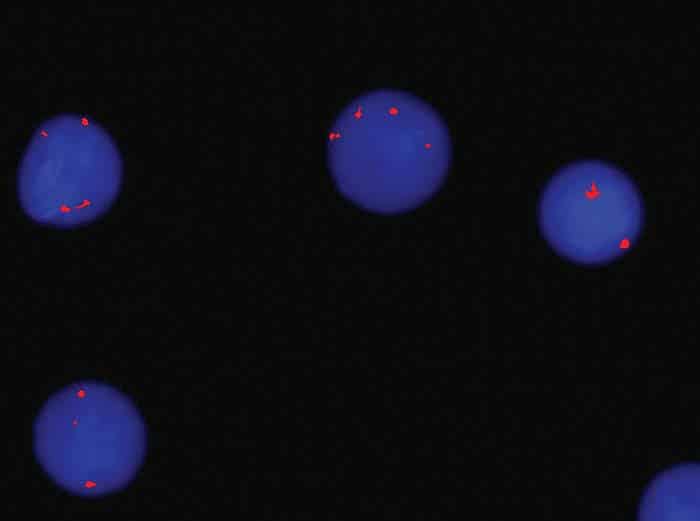
Fig. 3a. FISH trisomy 8
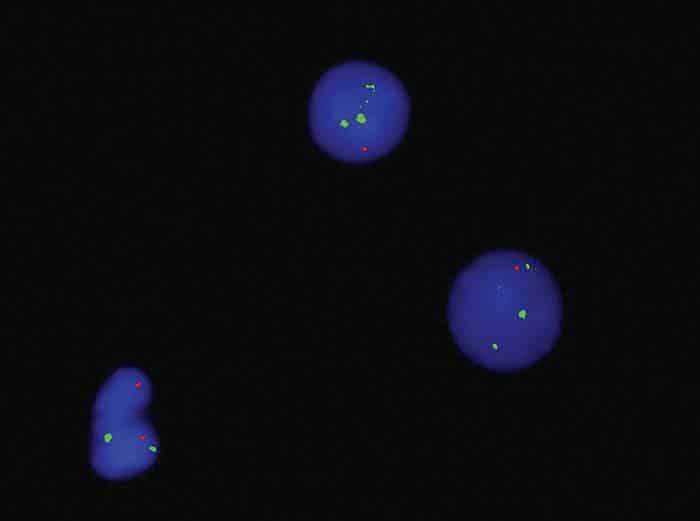
Fig. 3b. FISH 5q Del
Deletion of the long arm of chromosome 5 has a favorable prognostic implication in myelodysplastic syndrome but an unfavorable implication in AML. It often involves the loss of genetic material, impacting critical genes involved in hematopoiesis and cell cycle regulation. The 5q deletion was detected by FISH but not by conventional cytogenetics in this patient. Cryptic deletions, which may not be readily apparent under standard cytogenetic analysis, may pose diagnostic challenges but are increasingly recognized with advanced genomic techniques. These deletions are associated with distinct clinical and prognostic implications, influencing the disease course and treatment outcomes. The identification of cryptic 5q deletions is crucial for risk stratification, potentially guiding therapeutic decisions and possibly tailoring treatment strategies, such as the use of novel targeted therapies.4 Despite harboring this unfavorable chromosomal change, our patient initially responded well to azacitidine and showed improvement in his blood counts with clearance of blast cells in the peripheral blood.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
