Although it may seem that the initial best option would be to use RT-PCR testing primarily, each testing modality has its own advantages. FISH carries the advantage of there being specific probes to detect the translocation and can often be completed within 24 hours. In addition, break-apart probes can be used to identify fusions with other partners, an advantage when dealing with variant translocations. The disadvantages of FISH are its insensitivity for minimal residual disease monitoring and the possibility of missing cryptic cases, such as this case. Conventional karyotype, with a longer turnaround time of five to 10 days, can identify the usual t(15;17) and may also identify other genetic abnormalities that have prognostic significance. Karyotype may also miss cryptic translocations and it too is insensitive to monitoring for minimal residual disease. RT-PCR is by far the most sensitive testing modality and is frequently used for minimal residual disease monitoring and to identify cryptic translocations. Turnaround time, however, is typically longer than for FISH. RT-PCR has the added advantage that it can identify the specific translocation subtype, including bcr-1, -2, or -3.
A final option for identifying genetic abnormalities in such cases is next-generation sequencing. It is by far the most sensitive method of identifying genetic abnormalities because it can identify numerous abnormalities, not limited to cryptic translocations.9 Historically, the clinical use of next-generation sequencing has been limited due to high cost and long turnaround time; however, with technology advances the cost and turnaround time have been significantly decreased. Even with these advances, next-generation sequencing is not readily available in every laboratory.
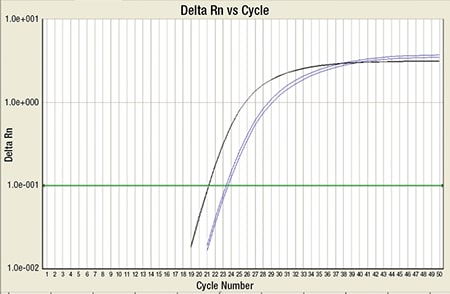
Fig. 7. RT-PCR for PML-RARA (blue) along with internal control ABL (black): This shows high copy number of PML-RARA, which crosses the cycle threshold (green line).
In cases where APL is suspected it is important to obtain rapid turnaround FISH and to alert the clinical team of the suspected diagnosis so ATRA therapy can be initiated promptly to prevent DIC. If FISH is demonstrated to be negative in a case with typical APL morphology and immunophenotype, it is important to perform additional molecular testing to rule out cryptic translocation before assigning a diagnosis other than APL. With appropriate diagnosis and treatment, patients with APL have an excellent prognosis.
- Paulson K, Serebrin A, Lambert P, et al. Acute promyelocytic leukaemia is characterized by stable incidence and improved survival that is restricted to patients managed in leukaemia referral centres: a pan-Canadian epidemiological study. Br J Haematol. 2014;166(5):660–666.
- Salomoni P, Dvorkina M, Michod D. Role of the promyelocytic leukaemia protein in cell death regulation. Cell Death Dis. 2012;3:e247.
- Pandolfi PP. Oncogenes and tumor suppressors in the molecular pathogenesis of acute promyelocytic leukemia. Hum Mol Genet. 2001;10(7):769–775.
- Choppa PC, Gomez J, Vall HG, Owens M, Rappaport H, Lopategui JR. A novel method for the detection, quantitation, and breakpoint cluster region determination of t(15;17) fusion transcripts using a one-step real-time multiplex RT-PCR. Am J Clin Pathol. 2003;119(1):137–144.
- Adams J, Nassiri M. Acute promyelocytic leukemia: a review and discussion of variant translocations. Arch Pathol Lab Med. 2015;139(10):1308–1313.
- Campbell LJ, Oei P, Brookwell R, et al. FISH detection of PML-RARA fusion in ins(15;17) acute promyelocytic leukaemia depends on probe size. Biomed Res Int. Epub March 28, 2013. doi:10.1155/2013/164501.
- Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—a Europe Against Cancer program. Leukemia. 2003;17(12):2318–2357.
- Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. Revised 4th ed. Lyon, France: WHO Press; 2017.
- Welch JS, Westervelt P, Ding L, et al. Use of whole-genome sequencing to diagnose a cryptic fusion oncogene. JAMA. 2011;305(15):1577–1584.
Dr. Coffman is a pathology resident and Dr. Chabot-Richards is associate professor of hematopathology and molecular pathology—both in the Department of Pathology, University of New Mexico, Albuquerque. Dr. Chabot-Richards is also medical director, TriCore Reference Laboratories, Albuquerque. Dr. Menkhaus, formerly a pathology resident at UNM, is in the Department of Pathology, Memorial Hospital of Sheridan County, Sheridan, Wyo.
Test yourself: Here are three questions taken from the case report.
Answers are online now at www.amp.org/casereports and will be published next month in CAP TODAY.
1. Which of the following is true regarding acute promyelocytic leukemia?
a. Patients are always older.
b. Patients may present in DIC.
c. Diagnosis requires identification of t(8;21).
d. Patients always present with leukocytosis.
2. The long form, or bcr-1, breakpoint accounts for what percentage of acute promyelocytic leukemia cases?
a. 15–20 percent
b. 80–90 percent
c. 45–55 percent
d. 35–45 percent
3. What is an advantage of performing fluorescence in situ hybridization over reverse transcription-polymerase chain reaction?
a. Rapid turnaround time
b. Ability to detect cryptic translocations
c. More sensitive for minimal residual disease
d. Allows for subtyping of breakpoint
Test yourself answers for March 2019 case report
In the March 2019 issue was a case report, “FDA-approved DNA blood test for colorectal cancer prompts patient to undergo colonoscopy,” written by members of the Association for Molecular Pathology. Here are answers (in bold) to the three “test yourself ” questions that followed that case report.
1. The five-year survival for patients diagnosed with stage I CRC is closest to:
a. 10 percent
b. 35 percent
c. 50 percent
d. 90 percent
2. In the United States, the number of age-eligible individuals who are not compliant with guideline-recommended CRC screening is closest to:
a. 1 million
b. 1 million
c. 20 million
d. 100 million
3. The FDA-approved SEPT9 test is indicated for:
a. Symptomatic patients who refuse other CRC screening methods.
b. Postoperative surveillance in patients with stage II CRC.
c. Asymptomatic, average-risk, age-eligible patients who have refused other screening methods.
d. Predictive testing in asymptomatic patients with a family history of CRC.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
