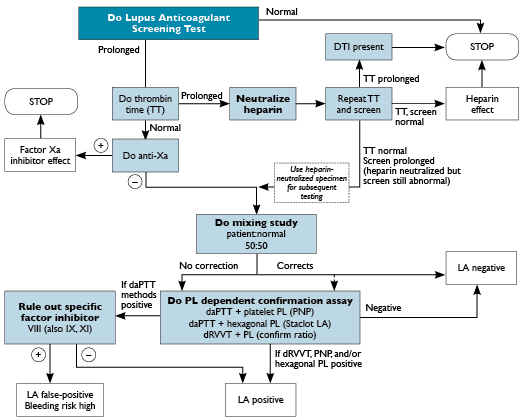
Fig. 24-1. There are many possible laboratory algorithms for diagnosis of the presence of a lupus anticoagulant (LA). Testing usually involves two phospholipid-dependent screening assays, such as the activated partial thromboplastin time with dilute phospholipid (daPTT), the dilute Russell viper venom time (dRVVT), or the dilute prothrombin time (dPT). If the screening test result is prolonged, a heparin, direct thrombin inhibitor (DTI), or factor Xa inhibitor effect should be excluded. This can be accomplished with the use of the thrombin time and anti-Xa assays combined with heparin removal/neutralization. If heparin is present, the sample can be tested further after heparin removal. If a DTI or factor Xa inhibitor is identified, testing should stop because these drugs cannot be neutralized and can result in false-positive lupus anticoagulant testing. | A mixing study, using a 50:50 mix of the patient’s plasma with normal pooled plasma, will usually not show correction with a lupus anticoagulant. Since the mixing study can falsely correct to normal with some LA, some laboratories proceed to the confirmatory step regardless of the mixing study result. Confirmatory testing involves demonstration of phospholipid (PL) dependence by repeating the abnormal screening assay with the addition of phospholipid and demonstrating a normalization of the clotting time. A factor inhibitor should also be excluded by performing factor assays.
Abbreviations: PL, phospholipid; PNP, platelet neutralization procedure.
Elevated factor VIII or baseline short clotting times might cause false-negative LA tests by preventing what would otherwise be a prolonged clotting time. A recent in vitro study found that significantly elevated C-reactive protein may cause false-positive lupus anticoagulant results with some aPTT-based screening and confirmatory methods, including the hexagonal phase assay. Severe, active infectious or inflammatory conditions can produce markedly elevated CRP, and this may explain some cases of transient positive LA.
Testing for a Lupus Anticoagulant. Lupus anticoagulants prolong various phospholipid-dependent clotting times because lupus anticoagulants are antiphospholipid antibodies, and phospholipid is essential for several steps in the coagulation cascade. If LA is present, it binds to phospholipid-protein complexes in the test tube, thereby interfering with the coagulation cascade and prolonging the clotting times. A testing algorithm for laboratory diagnosis of the presence of LA is shown in Figure 24-1 and is described below.
The most commonly used screening test for LA is an aPTT-based method. Laboratories can make their own sensitive aPTT reagent, for example, using the Bell and Alton extract, but most use a commercial LA-sensitive aPTT product. The diversity of reagent (quantity and quality of phospholipid) and instrument combinations is responsible for substantial variability in aPTT sensitivity for LA.
When the screening aPTT is prolonged, and the presence of an anticoagulant such as heparin, DTI, or Xa inhibitor is ruled out, a 50:50 mix with normal pooled plasma can be performed next. Often, mixing studies are performed before and after incubation of the mixture of the patient’s plasma with normal pooled plasma at 37°C to distinguish an LA from a specific factor inhibitor. Lupus anticoagulants typically show maximal inhibitory effect immediately upon mixing the patient’s plasma with normal plasma, whereas factor V and factor VIII inhibitors show maximal inhibitory effect only after prolonged incubation. The performance and interpretation of mixing study assays has been covered in detail in chapter 6, Coagulation Testing. If the aPTT is not adequately corrected (there is no consensus on the criteria for evaluating 50:50 mix results), a confirmation step is performed by repeating the aPTT with addition of extra phospholipid or by performing the platelet neutralization procedure (PNP).
The PNP is performed by repeating the aPTT with a platelet lysate as a source of phospholipids; if a lupus anticoagulant is present, the aPTT should become shorter as a result of binding by the excess membrane phospholipids. Platelet lysate preparations are available commercially or may be prepared from outdated platelets from the blood bank, provided the process and assay are validated. To prepare the lysate, platelets are centrifuged at 197g to remove red blood cells and washed three times with Tris-buffered saline. The platelet count is adjusted to 200,000 to 300,000 platelets/µL, and aliquots are frozen at -20°C. To perform the PNP, a platelet lysate aliquot is thawed and two aPTTs are performed in parallel: (1) lysate aPTT performed with 0.1 mL aPTT reagent + 0.1 mL test plasma + 0.1 mL frozen platelet suspension/excess phospholipid; and (2) saline aPTT performed with 0.1 mL aPTT reagent + 0.1 mL test plasma + 0.1 mL normal saline. The mixtures are incubated at 37°C for 5 minutes, 0.1 mL 25 mM CaCl2 is added, and clotting times are determined. The PNP difference (delta) is calculated as follows:
Each laboratory should determine an upper limit for the delta based on a local reference population for each lot of platelet lysate; if a patient’s delta exceeds the cutoff, this is indicative of phospholipid dependence.
 The hexagonal phase phospholipid neutralization (Staclot LA; Diagnostica Stago, France) is a commonly used and sensitive commercial aPTT-based LA test that combines screen, mix, and confirm steps. The source of extra phospholipid is a hexagonal phase phospholipid. Some laboratories start with a screen step, not required by the manufacturer, in which an aPTT is performed with the LA-sensitive phospholipid activator provided with the kit, and a positive cutoff is determined from a reference population. The mix-and-confirm step requires preparation of two tubes containing a 50:50 mix of test plasma and normal pooled plasma (provided in the kit, with polybrene to neutralize heparin). Buffer is added to tube 1, and a phospholipid (phosphatidylethanolamine) extract from soybeans that retains a hexagonal structure and avidly binds antiphospholipid antibodies is added to tube 2. After incubation, a lupus-sensitive aPTT reagent is added to each tube, followed by CaCl2, and clotting times are determined. If a lupus anticoagulant is present, a shorter aPTT should be obtained with the addition of hexagonal phase phospholipid to tube 2, and the difference (delta), calculated as ∆ = tube 1 aPTT − tube 2 aPTT, will be positive. The manufacturer recommends a cutoff of ≥8 seconds as diagnostic for the presence of LA when testing is performed on a manual ST4 (Stago) instrument; however, laboratories should establish their own lot-specific reference ranges for the assay. When the test is used on other platforms, an instrument-specific cutoff should be determined. False-positive Staclot LA results have been reported when a moderate or strong factor VIII inhibitor is present, which could delay recognition of a serious acquired bleeding disorder.
The hexagonal phase phospholipid neutralization (Staclot LA; Diagnostica Stago, France) is a commonly used and sensitive commercial aPTT-based LA test that combines screen, mix, and confirm steps. The source of extra phospholipid is a hexagonal phase phospholipid. Some laboratories start with a screen step, not required by the manufacturer, in which an aPTT is performed with the LA-sensitive phospholipid activator provided with the kit, and a positive cutoff is determined from a reference population. The mix-and-confirm step requires preparation of two tubes containing a 50:50 mix of test plasma and normal pooled plasma (provided in the kit, with polybrene to neutralize heparin). Buffer is added to tube 1, and a phospholipid (phosphatidylethanolamine) extract from soybeans that retains a hexagonal structure and avidly binds antiphospholipid antibodies is added to tube 2. After incubation, a lupus-sensitive aPTT reagent is added to each tube, followed by CaCl2, and clotting times are determined. If a lupus anticoagulant is present, a shorter aPTT should be obtained with the addition of hexagonal phase phospholipid to tube 2, and the difference (delta), calculated as ∆ = tube 1 aPTT − tube 2 aPTT, will be positive. The manufacturer recommends a cutoff of ≥8 seconds as diagnostic for the presence of LA when testing is performed on a manual ST4 (Stago) instrument; however, laboratories should establish their own lot-specific reference ranges for the assay. When the test is used on other platforms, an instrument-specific cutoff should be determined. False-positive Staclot LA results have been reported when a moderate or strong factor VIII inhibitor is present, which could delay recognition of a serious acquired bleeding disorder.
The dilute Russell viper venom time is one of the most commonly performed LA screening tests. Russell viper venom activates factor X to initiate the common coagulation pathway. “Dilute” indicates that a dilute (minimal) amount of phospholipid is present, and phospholipid is required to form the prothrombinase complex that converts prothrombin into thrombin. If LA is present, it should bind to the phospholipid, thereby prolonging the dRVVT clotting time. The major advantage of the dRVVT method is that it is unaffected by alterations in the extrinsic or intrinsic coagulation pathways (inhibitors, coagulopathies, elevated factor VIII activity). However, common pathway factor deficiencies or inhibitors, excess heparin, and direct thrombin inhibitor or factor Xa inhibitor anticoagulants can produce false-positive results.
The variable sensitivity of dRVVT methods used to screen for LA is due primarily to different sources and concentrations of Russell viper venom and phospholipid reagents. Commercial dRVVT kits provide reagents for both LA screen and confirm steps and typically contain a heparin-neutralizing agent. Typically, the dRVVT assay employs three phases: (1) dRVVT screen, (2) dRVVT mix, and (3) dRVVT confirm. At each step, results are commonly reported as a ratio of the patient’s plasma clotting time divided by the clotting time of NPP. The cutoff for a positive dRVVT screen ratio is greater than twice the standard deviation (SD) of the dRVVT ratio mean from a reference population (>mean+2SD). If the screen is positive, the dRVVT mix is performed using a 50:50 mixture of patient plasma and normal pooled plasma (NPP). A dRVVT mix ratio is calculated as follows:

A typical cutoff for a positive mix ratio is greater than twice the SD of the mean of the ratio of a reference population. If the screen and mix ratios are both positive, first the dRVVT confirm ratio step is performed using added phospholipid. The dRVVT confirm ratio is calculated as follows:

Then a normalized dRVVT ratio is calculated using the screen ratio in the numerator and the confirm ratio in the denominator:

The cutoff for the normalized ratio is greater than twice the SD of the mean of the normalized ratio of a reference population. Commercial dRVVT kits vary in their sensitivity for detecting LA. Laboratories should review the literature and proficiency testing survey results and select a sensitive one.
The kaolin clotting time (KCT) was first described as a screen for LA in 1978 by Exner et al. It is considered to be one of the most sensitive LA screen methods and continues to be popular in European hemostasis laboratories, although current guidelines prefer aPTT-based and dRVVT-based methods as the first-line tests. As with aPTT-based tests, the KCT is based on the principle that activation of factor X and prothrombin on the surface of residual phospholipid in platelet-poor plasma is markedly sensitive to interference from LA antibodies. The activator (kaolin) is added to different ratios of platelet-poor test plasma and normal pooled plasma without an exogenous source of phospholipid, and clotting times are monitored. The delta KCT is a variation of the original method. Two preparations of test plasma are used: diluted 1:4 with normal pooled plasma and undiluted. In step 1, 0.2 mL of 1:4 or undiluted test plasma is combined with 0.1 mL 2% kaolin and incubated for 3 minutes. In step 2, 0.2 mL of 25 mM CaCl2 is added and clotting time is measured. Then, the following calculation is made:

A typical cutoff value for LA is a delta greater than 14 seconds. The KCT method is an extremely sensitive screening test for most lupus anticoagulants, is inexpensive, and can be automated. The KCT is also exquisitely sensitive to residual platelets, which led some to filter (0.2-μm filter) the platelet-poor plasma before testing, or before freezing for later testing, to prevent false-negative results. However, the use of filters is not recommended by current CLSI guidelines.
Another “in-house” or commercially available LA screening technique is the dilute prothrombin time (dPT), also known as the tissue thromboplastin inhibition or thromboplastin dilution test. Routine prothrombin time (PT) reagents contain a large amount of phospholipid, such that PT clotting times are typically normal in the presence of LA. Performing a PT with a diluted commercial thromboplastin (range 1:50 to 1:1000) enhances sensitivity to the presence of a lupus anticoagulant by diluting the amount of phospholipid. In other words, LA can prolong the clotting time of a dPT reagent, whereas undiluted PT reagents usually contain too much phospholipid for LA to interfere. However, the sensitivity of dPT LA screen is dependent on the type (recombinant human vs rabbit or bovine tissue-derived) and brand of thromboplastin, the coagulation instrumentation, and, most important, the dilution factor that is used. Because there is no accepted standard method, the reported sensitivity of the dPT LA screen is variable.
Exclusion of Other Abnormalities (Factor Inhibitors). Certain inhibitors can cause false-positive LA tests. Conversely, lupus anticoagulants can cause false-positive Bethesda assays for factor VIII inhibitor quantification. If LA tests are positive, efforts should be made to ensure that a different type of inhibitor is not present. This should include excluding the possibility of heparin, a direct thrombin inhibitor (such as dabigatran, argatroban, bivalirudin, or hirudin), or a direct factor Xa inhibitor, as described above. If the routine aPTT and PT are normal, the laboratory can be reasonably sure that a specific inhibitor (such as a factor VIII inhibitor) is not present. If the routine aPTT is prolonged and no anticoagulants are present, a factor VIII assay should be considered to ensure that a factor VIII inhibitor is not present. Assays for factors IX and XI can also be considered if the factor VIII activity is normal. If the PT is prolonged and no anticoagulants are present, assays for factors II, V, VII, X, and fibrinogen can be considered.
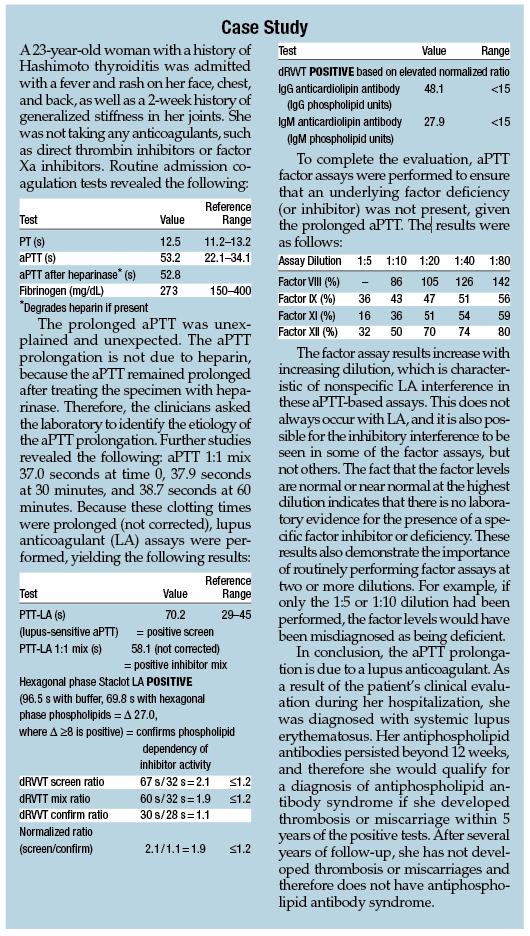
Three or more dilutions should be used in the factor assays. If a lupus anticoagulant, heparin, factor Xa inhibitor, or direct thrombin inhibitor is present, the measured factor level will be lower at the lower dilution (eg, 1:10) than at a higher dilution (eg, 1:20). If a specific factor inhibitor is present, the activity of the targeted factor is usually less than 10% with minimal increase upon dilution, and more normal results are typically obtained for other factors (although the other factor assays may show a mild increase upon dilution, ie, weaker specific inhibitor effect; see the example factor assays in the case study presented in this chapter).
If a markedly low factor VIII is found in a patient who also tests positive for LA, a chromogenic factor VIII assay might be useful to distinguish between a true decrease in factor VIII due to a factor VIII inhibitor versus an unusually strong interference in the factor VIII assay due to the LA. Lupus anticoagulants do not interfere with chromogenic factor VIII assays; therefore, if the low factor VIII activity (by the routine aPTT-based assay) is due to LA interference, the chromogenic factor VIII result will be normal. If, on the other hand, the low factor VIII activity in the routine aPTT-based assay is due to a true decrease resulting from a factor VIII inhibitor, the chromogenic factor VIII result will also be low. Rare patients may demonstrate both LA and factor VIII inhibitor activity.
When Results Do Not Agree. When one LA confirmatory test is positive but another is negative (for example, positive hexagonal phase with negative dRVVT), the overall interpretation is that the patient is positive for LA, because no single test is 100% sensitive for LA. However, possible causes of false-positive results in the positive assay should be considered, and it is recommended that the test be repeated 12 or more weeks later with the same method to determine if the LA is persistent. When the mixing step is positive but the confirm step is negative, the results should be considered negative for LA, and other causes for prolonged mixing studies should be investigated. Lastly, if the mixing step is negative (corrects to normal) while the phospholipid confirmatory step is positive, the results are positive for LA, if reasons for false-positive confirmatory results can be excluded. It is possible that a mixing study can be falsely negative because of dilution of the LA.
Due to the complexities of lupus anticoagulant testing, results are best accompanied by an interpretation by a pathologist or other qualified expert.
Monitoring Anticoagulation in Patients With Lupus Anticoagulants
Lupus anticoagulants commonly prolong the aPTT. When patients with LA are treated with heparin, monitoring heparin with the aPTT can be difficult because it is not known how much of the prolongation is due to heparin and how much is due to the LA. Even if the aPTT is normal at baseline, it is possible that the LA will prolong the aPTT beyond what is expected when heparin is added. One approach to such patients is to treat them with low-molecular-weight heparin, which is not monitored with the aPTT and has a more predictable dose-response relationship. If low-molecular-weight heparin is contraindicated and heparin must be used, a chromogenic heparin assay (anti-factor Xa assay) can be performed. If heparin assays are performed, it is suggested that aPTT also be measured on the same specimen to help assess which aPTT values are within the therapeutic range for the patient, because aPTT tests are usually much more readily available.
It is less common that LA may prolong the PT, depending on the PT reagent. When patients with LA are treated with warfarin, monitoring can occasionally be difficult because the PT/INR (prothrombin time/international normalized ratio) might be prolonged by the lupus anticoagulant, even if the PT is normal at baseline. One approach is to measure a chromogenic factor X level when the INR has reached the therapeutic range. A clot-based factor X activity can probably suffice if a chromogenic factor X assay is not available, but the LA, in theory, could interfere with the PT-based factor X assay. The laboratory should establish the range of factor X values that correspond to an INR of 2 to 3 among patients on stable warfarin doses. An approximation is that an INR of 2 to 3 corresponds roughly to chromogenic factor X values of 20% to 40% and PT-based factor X values of 5% to 15%.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
