Amy Carpenter Aquino
March 2023—Assessing cardiac allograft rejection from endomyocardial biopsy and assigning a differential diagnosis to cancers of unknown origin have been shown to get a boost from AI-driven computational pathology models. So too has identifying subregions of high diagnostic value on whole slide images.
In addition, an algorithm published late last year addresses familiar challenges in whole slide image search: speed, accuracy, and scalability.
Faisal Mahmood, PhD, spotlighted these computational pathology models when he spoke at the Association for Molecular Pathology meeting last November. Dr. Mahmood, associate professor in the Department of Pathology at Harvard Medical School, and in the Division of Computational Pathology at Brigham and Women’s and Massachusetts General hospitals, shed light on what the field of computational pathology is aiming now to do.
His group studies phenotypic and morphologic data and performs quantitative spatial analysis to provide early diagnoses and prognoses, predict response to treatment, stratify patients, and discover biomarkers. They also look at integrative sources that are already quantitated, such as multimodal or molecular biomarkers, and use deep learning to integrate the information. “We’re also interested in genotypic and phenotypic responses to disease,” says Dr. Mahmood, who is also a member of the Cancer Data Science Program at Dana-Farber Cancer Institute and of the Cancer Program at Broad Institute of Harvard and MIT.
Conventional algorithms are difficult to use with typically large pathology images, he noted. As departments move to digital pathology-based workflows, “our hope is we will have a wealth of data” and “be able to do things we were never able to do before—rediscover diseases we already knew about and discover new ones, discover new morphologic features, correlate the morphology with molecular information in a more holistic manner.”
While supervised learning methods require manual labeling or region of interest extraction, deep learning does not require feature engineering, “so you don’t need to use existing human knowledge to handcraft very fine details within the image,” Dr. Mahmood said. “Annotations are enough.”
But annotating whole slide images is time-consuming and “far apart from what a clinical workflow looks like,” he said. The solution: weakly supervised learning, which requires only information that is already on WSIs and slide-level labels from pathology reports. Graph computational networks and multiple-instance learning are two common approaches.
Dr. Mahmood and colleagues in 2021 reported on a weakly supervised deep-learning method for data-efficient whole slide image processing and learning that requires only slide-level labels (Lu MY, et al. Nat Biomed Eng. 2021;5[6]:555–570). Their AI-driven method, called clustering-constrained-attention multiple-instance learning (CLAM), “uses a number of different bells and whistles from conventional machine learning and brings them together and applies them to pathology images,” he said. CLAM uses attention-based learning to identify the most important subregions within a WSI—to accurately classify the whole slide—and uses “tricks,” he said, to deal with data efficiency and imbalances. It also makes use of powerful pretrained ResNet encoders: “We use features learned on real-world images and then apply them to pathology images,” Dr. Mahmood explained.
The CLAM method segments and patches a WSI into smaller patches. “Once we have smaller patches, we extract features in a lower dimensional representation,” he said. An initial study used the conventional ResNet-50 encoder pretrained on real-world images, using just the first few blocks. “From there we learned more common, basic features of an image.” The intention, he said, was to learn what the most important regions are within the WSI. The model used N parallel attention branches that calculate N unique slide-level representations to enable multiclass classification. “Pathology in general is multiclass, and there could be many, many findings” in a WSI, he said.
“Then we rank the patches,” Dr. Mahmood said, and the patches are then pooled based on their learned rank to get to the slide-level representation. “The trick we use is instance-level clustering”—clustering similar morphologic images within the WSI. “Because we have attention built in and can rank the patches, we can project that back onto the whole slide image as an interpretability mechanism to make it a little more transparent than what features the model uses in making these classification determinations,” he said.
In the initial study, his group applied the model to renal cell cancer subtyping, non-small cell lung cancer subtyping, and detection of breast cancer lymph node metastasis. For the breast cancer lymph node metastasis, the WSI—“we used about 1,000 images”—was adapted to an independent cohort from Brigham and Women’s Hospital. He and his coauthors reported strong performance and the ability to generalize to independent test cohorts and varying tissue content. They write, “Our analysis demonstrated that CLAM can be used to train interpretable, high-performance deep-learning models for both binary and multi-class WSI classification using only slide-level labels without any additional annotation.”
Said Dr. Mahmood, “As we go from using about 700 slides to train this all the way down to 100 slides, there’s a drop in performance, but the drop in performance is not that large for this particular method, which means this approach, by using whole slide images, just labels that are available in pathology reports, and data efficiency, can be used for a lot of downstream tasks.”
To test how far they could push the model’s adaptability, Dr. Mahmood’s group tested CLAM with images taken with a cell phone connected to a brightfield microscope. “We found it was possible,” though there was an expected drop in performance in non-small cell lung cancer subtyping and renal cell carcinoma subtyping.
“So now that we had a mechanism to train these models on whole slide images using slide-level labels and doing it in an easy, effective manner, we planned to target the problem of cancer of unknown primary origin,” Dr. Mahmood said.He and colleagues questioned whether it was possible to use conventional WSIs to predict tumor origin. They trained their new deep-learning–based algorithm using 22,833 WSIs from cases with known primary origin from The Cancer Genome Atlas and Brigham and Women’s Hospital, and from MGH in subsequent versions. The deep-learning algorithm—tumor origin assessment via deep learning (TOAD)—was then tested on an internal cohort of 6,499 primary and metastatic cases, Dr. Mahmood said. They had an external test cohort of 682 cases from 223 medical centers. And they assessed their model using an additional test data set of 317 cases (from 152 cen-ters) of cancer of unknown primary that were assigned a primary differential based on ancillary tests, radiology, history, clinical correlation, or at autopsy (Lu MY, et al. Nature. 2021;594[7861]:106–110).
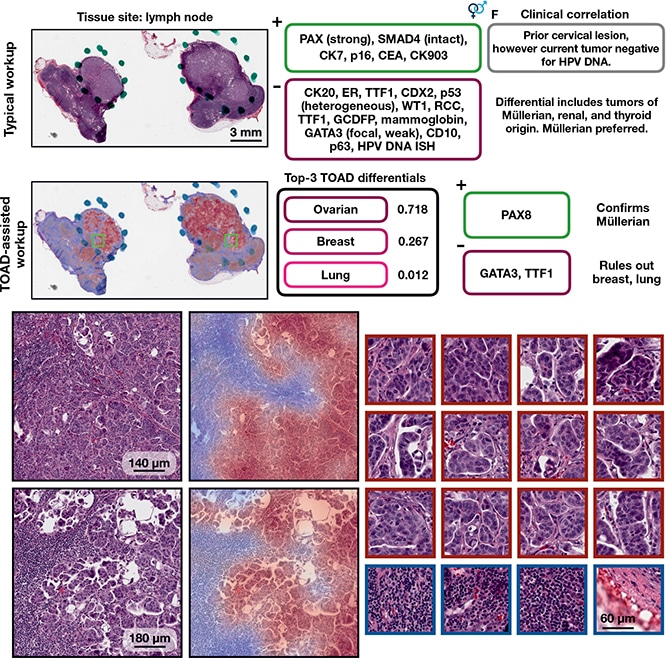
TOAD-assisted CUP workup: example 1. Top, a representative case that underwent a standard CUP workup involving extensive IHC staining and clinical correlation. Strong PAX8 staining suggested a Müllerian origin and multiple IHC tests were used to rule out other primary tumors. Retrospectively, they analyzed the case with TOAD and found that the top-3 determinations were ovarian, breast, and lung, and, after this determination, that only three IHC stains (PAX8, GATA3, and TTF1) needed to be used to confirm a Müllerian origin and rule out breast carcinoma and lung adenocarcinoma. This workflow demonstrates how TOAD can be used as an assistive diagnostic tool. Bottom, medium magnification and corresponding heat maps of representative areas of tumor, with high-magnification, high-attention patches on the right outlined in crimson and low-attention patches outlined in navy. Ming Y. Lu et al. AI-based pathology predicts origins for cancers of unknown primary. Nature. 2021;594(7861):106–110, Springer Nature.
The TOAD architecture was similar to the CLAM architecture but it was posed as a multitask problem, Dr. Mahmood said. “The model can predict whether the case is primary or metastatic and assign possible origins” for use in diagnosis, research, and guiding downstream ancillary testing. And “it did quite well on both internal and external test sets,” he said. “Wherever the model was more confident, it would make more accurate predictions.” On the test set of 6,499 (not seen by the model during training), it achieved an overall accuracy of 83.4 percent. He and his coauthors write, “When the model is evaluated using top-k differential diagnosis accuracy—that is, how often the ground truth label is found in the k highest confidence predictions of the model—TOAD achieved a top-3 accuracy of 95.5% and top-5 accuracy of 98.1%.”
“The top-three and top-five predictions were very high,” Dr. Mahmood said, “which means the model could be used to tell what the possible origins are and subsequently to order IHC tests or other ancillary tests” based on that information.
With limited time to follow all patient outcomes, the study authors divided the 317 cases with a primary differential into 193 cases with high certainty differential and 124 cases with low certainty differential and found 61 percent of cases agreed with the model’s prediction. The top-three agreement was 82 percent; the top-five agreement was 92 percent. “The top-three and top-five predictions were still very, very high,” Dr. Mahmood said.
More recently, his group has been trying to integrate more information for origin prediction. “We’ve found in general that integrating both histology and genomics has improved the prediction of origins across the board within the bounds of the study,” he said, noting they’re working now on expanding it. “We’re also able to explicitly go in and look at what morphologic origins are used in the whole slide image and then what’s used and what’s important within the molecular profile for each one of the origins we have used.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
