Stuart E. H. Cameron, MD
Sinchita Roy-Chowdhuri, MD, PhD
August 2023—Accurate and timely diagnosis is the cornerstone of effective patient care, particularly in the field of pulmonary pathology. To address the challenges health care professionals face in diagnosing and reporting respiratory conditions, the International Academy of Cytology, together with the International Agency for Research on Cancer, recently developed the World Health Organization Reporting System for Lung Cytopathology in an attempt to standardize the reporting of lung cytopathology to enable clinicians to make informed decisions and improve patient outcomes. This reporting system provides a standardized framework for interpreting and reporting pulmonary cytology specimens, and for the first time provides an international system that recognizes the variation in the availability of ancillary testing globally to provide options for patient management that can be used across all medical settings. It includes guidance for optimized specimen sampling and processing techniques and best practice applications of ancillary testing.
In this article, we explore the highlights of the new WHO Reporting System for Lung Cytopathology and provide case examples that illustrate its practical application.
Pulmonary cytology plays a crucial role in diagnosing various respiratory diseases, including lung cancer, infections, and interstitial lung diseases. However, interpreting cytology specimens accurately can be complex and subjective, leading to diagnostic challenges and potential errors. The WHO Reporting System for Lung Cytopathology emphasizes the importance of high-quality specimen collection and preparation. Techniques such as bronchoscopy, transthoracic fine-needle aspiration, and endobronchial ultrasound-guided sampling are discussed, as are optimal sample preservation and processing methods.
A significant advancement in the new WHO reporting system is the adoption of standardized terminology for reporting cytology findings to foster consistency and enhance diagnostic accuracy. This allows for better communication between pathologists and clinicians, enabling effective treatment planning.
In the new system, the risk of malignancy has been stratified into five categories: insufficient/inadequate/nondiagnostic, benign, atypical, suspicious for malignancy, and malignant (Table 1). These categories are linked to clinical management options for further workup and management, based on the availability of medical resources and local practices.
The reporting system also recognizes the increasing role of ancillary testing in lung cancer diagnosis and provides recommendations for integrating ancillary techniques, such as immunocytochemistry and molecular testing, into cytology practice. This integration of molecular cytopathology allows for the identification of specific oncogenic alterations and facilitates personalized treatment approaches such as targeted therapy and immunotherapy.
To illustrate the practical application of the WHO Reporting System for Lung Cytopathology, let us consider two case examples.

Table 1. World Health Organization international system for reporting lung cytopathology on FNAB: implied risk of malignancy and clinical management options by diagnostic category. [FNAB, fine-needle aspiration biopsy; CLIN, clinic; IMG, imaging; MICRO, microbiology; CNB, core needle biopsy; MDT, multidisciplinary team; ROSE, rapid on-site evaluation. Reprinted from WHO Reporting System for Lung Cytopathology, vol. 1, 1st ed. IAC-IARC-WHO International Joint Editorial Board, chapter 2, page 12, 2022. ( Available at https://tumourclassification.iarc.who.int )]
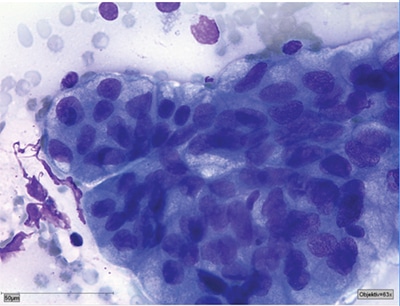
Reprinted from WHO Reporting System for Lung Cytopathology, vol. 1, 1st ed. IAC-IARC-WHO International Joint Editorial Board, chapter 7, 2022.
Case 1 sample report:
Type of specimen: Transthoracic needle aspiration (TTNA).
Clinical and imaging information: 60-year-old male with persistent cough, weight loss, and hemoptysis. Heterogeneous, FDG-avid 2.5-cm right lung mass with poorly defined borders.
Diagnostic summary/conclusion:
- Category: MALIGNANT
- Diagnosis: Adenocarcinoma
- Microscopic description: Cellular smears with malignant cells forming acinar groups with high N:C ratio, visible nucleoli, and moderate amounts of vacuolated cytoplasm.
- Comment: Tumor cells are TTF-1 and napsin A positive; P40, CK5/6 negative. Please also refer to the concurrently acquired needle core biopsy.
Case 2. A 45-year-old female with a history of recurrent respiratory infections presents with a productive cough, fever, and shortness of breath. Bronchoscopy with bronchoalveolar lavage is performed, and cytology reveals numerous neutrophils, lymphocytes, and Gram-positive cocci. Following the new WHO reporting system, the cytopathologist classifies the tumor into the benign category, with correlation with imaging findings and microbiologic studies. An infectious etiology of Streptococcus pneumoniae is identified, and prompt initiation of appropriate antibiotics leads to resolution of symptoms and the patient’s recovery.
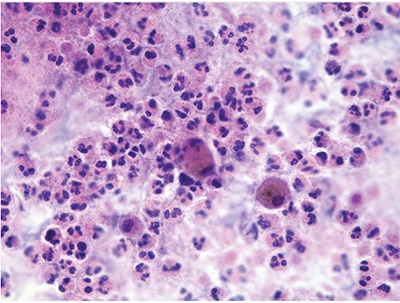
Reprinted from WHO Reporting System for Lung Cytopathology, vol. 1, 1st ed. IAC-IARC-WHO International Joint Editorial Board, chapter 4, 2022.
Case 2 sample report:
Type of specimen: Bronchoalveolar lavage (BAL).
Clinical and imaging information: 45-year-old female with recurrent respiratory infections presents with a productive cough, fever, and shortness of breath.
Diagnostic summary/conclusion:
- Category: BENIGN
- Diagnosis: Acute inflammation with bacteria
- Microscopic description: Cytology preparations with numerous neutrophils, lymphocytes, and Gram-positive cocci.
- Comment: Correlation with imaging findings and microbiologic studies recommended.
In summary, the WHO Reporting System for Lung Cytopathology provides standardized reporting terminology for lung cytopathology with guidance on specimen collection, processing, diagnostic categories, and ancillary testing integration, all of which facilitates better communication and improves patient care decisions.
- IAC-IARC-WHO International Joint Editorial Board. WHO Reporting System for Lung Cytopathology. 1st ed. World Health Organization; 2022. IAC-IARC-WHO Cytopathology Reporting Systems; vol. 1.
- WHO Classification of Tumours Editorial Board. Thoracic Tumours. World Health Organization; 2021. WHO Classification of Tumours; 5th ed., vol. 5.
- Layfield LJ, Baloch Z, Elsheikh T, et al. Standardized terminology and nomenclature for respiratory cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol. 2016;44(5):399–409.
- Schmitt FC, Bubendorf L, Canberk S, et al. The World Health Organization reporting system for lung cytopathology. Acta Cytol. 2023;67(1):80–91.
- Yoshizawa A, Hiroshima K, Takenaka A, et al. Cytology reporting system for lung cancer from the Japan Lung Cancer Society and the Japanese Society of Clinical Cytology: an extensive study containing more benign lesions. Acta Cytol. 2022;66(2):124–133.
- Canberk S, Field A, Bubendorf L, et al. A brief review of the WHO reporting system for lung cytopathology. J Am Soc Cytopathol. 2023;12(4):251–257.
Dr. Cameron, of Minneapolis, Minn., is a member of the CAP Cytopathology Committee. Dr. Roy-Chowdhuri, a former member of the CAP Cytopathology Committee, is an associate professor in the Department of Pathology, University of Texas MD Anderson Cancer Center.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
