Michael Henry, MD
Janie Roberson, SCT(ASCP)
August 2023—Cytology gynecologic statistical laboratory data must be analyzed in the context of changing screening modalities and patient populations. This is especially important with the increasing use of primary high-risk HPV screening because diagnostic metrics will be markedly different in that patient population.
To ensure that national benchmarking data remain contemporary, the CAP Cytopathology Committee collected 2021 preparation type volumes and interpretive totals. Updated gynecologic cytology data are provided for conventional, SurePath, and ThinPrep preparations. The interpretation metrics were updated previously in 2020 based on 2019 data. There were no statistically significant distribution differences between the two time periods for any of the categories. These new rates will appear in the 2023 edition of the laboratory accreditation checklist. (See CYP.07600 with Pap 2021 gyn cytology metrics, below.)
To assess the effect of new technologies, the survey also included several new demographic questions designed to gauge laboratory practice related to both primary HPV screening and cotesting. Only 9.6 percent (n=22) of laboratories reported screening Pap test slides derived from primary HPV screening. Of those, only two laboratories reported that more than 25 percent of their slides were derived from positive HPV primary screening, and eight laboratories reported that more than 10 percent of their slides came from these patients. Of interest, 46.8 percent of respondents reported including diagnostic interpretations from positive primary HPV screening in their overall statistics for gynecologic cytology; 27.7 percent were unsure if these statistics were included.
While the reported inclusion of primary HPV-tested slides remains low, this emerging modality should be considered as statistical analysis is performed. A reflexed Pap test in which high-risk HPV is detected does not accurately reflect the laboratory’s HPV unknown screening population. As these high-risk-HPV-positive reflex cases are evaluated cytologically, the data will be skewed more toward SIL than in a general patient population. Ideally, these tests should be excluded from consideration as the laboratory makes comparisons to the CAP benchmarking data.
Because of this, data from the two laboratories with greater than 25 percent of their cases derived from reflexed high-risk HPV primary screening were not included in the 2023 checklist metrics. In 66 percent (n=259) of responding laboratories, cotesting was reported in more than 40 percent of Pap tests. Additionally, in 28.6 percent of laboratories, cytologists know the HPV result prior to screening cotest Pap tests, and in 43.9 percent of cases pathologists routinely know the HPV result prior to signing out cotest Pap tests. Whether interpreting Pap tests with knowledge of HPV test results introduces bias is yet to be determined.
In light of the increasing use of primary high-risk HPV screening, a statement has been added to the explanatory note for CYP.07600. It reads: “These benchmarking data may not be applicable for laboratories that utilize primary HPV screening for a significant portion of cervical cancer screening. Results were excluded for laboratories that included primary HPV screening results in the interpretive totals when more than 25% of their cervical/gynecologic slides were from positive primary HPV screening.”
Laboratories should be aware of the potential impact of changing screening modalities such as cotesting and primary HPV screening on overall diagnostic distributions for gynecologic cytology.
Dr. Henry is professor emeritus, Mayo Clinic, Rochester, Minn. Janie Roberson is senior director of laboratory quality, anatomic pathology, and cellular therapy, UAB Medicine, Birmingham, Ala. Both are members of the CAP Cytopathology Committee.
CYP.07600 Statistical Records—Gynecologic Cytopathology
For gynecologic cytopathology cases, statistical records are maintained and evaluated at least annually, and include the following:
- Total number of gynecologic cytology cases examined.
- Number of cases reported by diagnosis for each specimen type (including the number reported as unsatisfactory for diagnostic interpretation).
- Number of cases with a diagnosis of HSIL, adenocarcinoma, or other malignant neoplasm for which histology results were available for comparison.
- Number of cases where cytology and histology are discrepant.
- Number of cases where any rescreen of a normal or negative specimen results in reclassification as low-grade squamous intraepithelial (LSIL), HSIL, adenocarcinoma, or other malignant neoplasms.
- Number of negative cases rescreened before sign-out.
NOTE: The data must be evaluated by the laboratory director or designee and included in the annual cytopathology statistical report. Inclusion of AGC data is optional. Separate statistics for conventional and each type of liquid-based preparations are required.
The benchmarking data listed in the table are based on 2021 case volumes. These benchmarking data may not be applicable for laboratories that utilize primary HPV screening for a significant portion of cervical cancer screening. Results were excluded for laboratories that included primary HPV screening results in the interpretive totals when more than 25% of their cervical/gynecologic cytology slides were from positive primary HPV screening. In evaluating its statistics, the laboratory’s patient population should be taken into consideration. Percentile-reporting rates refer to the distribution of individual laboratory responses from reporting rates in various categories. Responses are ranked from lowest to highest, and the 50th percentile-reporting rate refers to the median response. A 25th percentile-reporting rate (which corresponds to 1.7% in the table) for the ThinPrep LSIL category means that a quarter of laboratories have LSIL rates of 1.7% or less. A 90th percentile-reporting rate (which corresponds to 11.7% in the table) for ASC-US in ThinPrep preparations means that 9 of 10 laboratories have an ASC-US rate of 11.7% or less.
The reporting rates for ASC-US, ASC-H, AGC, LSIL, HSIL, and UNSATISFACTORY are given as percentages of total case volume. An ASC-US rate of 2.0% means 2/100 cases in the lab are designated ASC-US. The ASC/SIL figure is a calculated ratio: the percentage or number of a laboratory’s ASC-US and ASC-H cases divided by the percentage or number of LSIL, HSIL, and malignant cases. A laboratory with 4% ASC cases and 3% SIL cases has an ASC/SIL ratio of 1.3, as compared to the median ASC/SIL ratio of 1.5 for conventional Paps, 2.0 for ThinPrep and 1.8 for SurePath.
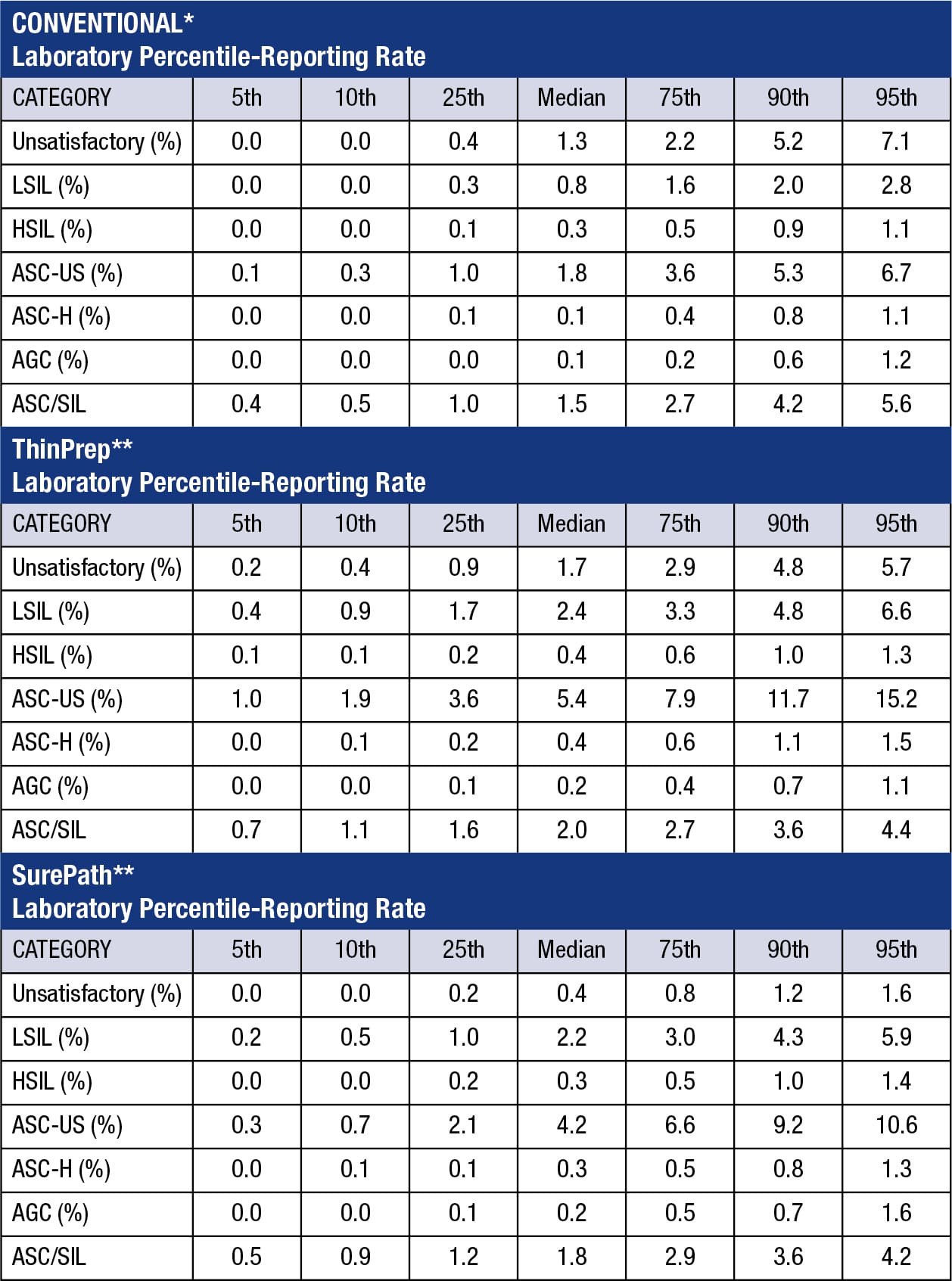
*Includes conventional annual test volume of >60. **Includes SurePath and ThinPrep annual test volume of >300.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
