The well-documented special stain used for GIM subtyping—i.e. high-iron diamine/Alcian blue stain—is not used routinely because of the toxicity of the reagents. The stain divides GIM into types I, II, and III, with type I corresponding to complete GIM and types II and III corresponding to incomplete GIM. Type III has the highest reported association with progression to gastric cancer, she said. “This type is characterized by its sulphomucin content, i.e. colon-type mucin, which stains the goblet cells and the background columnar cells brown.”
H&E may be the best stain for subtyping, she said, but the issue with H&E subtyping is that GIM is rarely fully complete or fully incomplete. “Mostly it’s composed of different proportions of complete and incomplete, and, yes, you can report it as ‘mixed predominantly complete’ or ‘mixed predominantly incomplete,’” for example, “but the cutoffs for the mixed types are arbitrary at this point.”
Adequate sampling is a major prerequisite for classifying GIM, Dr. Setia said. “And that is incumbent on your gastroenterologist to provide when they ask you for intestinal metaplasia classification.” The AGA and MAPSII guidelines again differ in their recommendations, with the AGA recommending the Sydney system five-biopsy protocol, and the MAPSII guidelines a four-biopsy protocol. The Sydney protocol indicates one biopsy from the incisura, two from the antrum—one from the lesser and one from the greater curvature—and two from the corpus (also from lesser and greater curvature). “While it seems a bit much, I’ve noticed that most of our gastroenterologists follow the Sydney protocol. So it’s not impossible to expect adequate sampling from our gastroenterologists.” This reflects the University of Chicago experience, she tells CAP TODAY, not necessarily the pattern in the community.
The MAPSII-recommended protocol involves obtaining two antral biopsies from the lesser and greater curvature, and two from the corpus, also from the lesser and greater curvature, and it’s in the 2019 version of the guidelines, not the 2012 version. “The change in recommendation was based on a large study of around 4,000 biopsies, where they found the yield of the four-biopsy protocol to be the same as the five-biopsy protocol,” Dr. Setia explained.
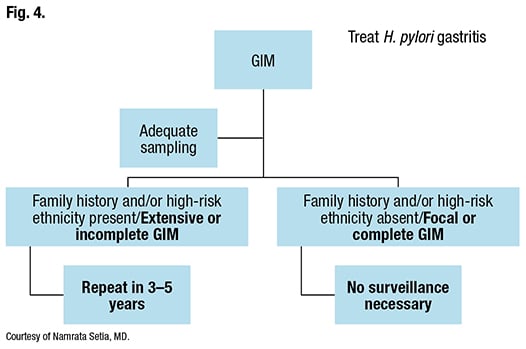
How should GIM be surveilled? It depends, Dr. Setia said, on whether sampling was adequate and if the patient is at high risk clinically or pathologically. By the AGA guidelines, the groups with higher risk clinically are racial and ethnic minorities with a known high gastric cancer incidence, immigrants from regions with a high incidence of gastric cancer, and those with a first-degree relative who has had gastric cancer. Pathologically, extensive or incomplete-type GIM is considered high risk.
Dr. Setia provided an example of how a patient with GIM should be surveilled and stratified, according to the AGA guidelines. “You see intestinal metaplasia in a patient’s antral biopsies, but because the body mucosa wasn’t sampled, sampling was inadequate. You sign it out as atrophic antral gastritis with intestinal metaplasia.” A gastroenterologist calls to ask what should be done next. Ask the gastroenterologist if the patient is at high risk for gastric cancer per the AGA guidelines, she said. If so, the AGA recommendation is to perform a repeat endoscopy within a year if the patient elects to do so, “to ensure you have an optimal baseline with adequate sampling.”
If sampling is adequate and the patient has high-risk clinical or pathologic features, the AGA’s conditional recommendation (low-quality evidence) is to perform a repeat upper endoscopy with careful mucosal visualization in three to five years, if the patient elects to do so. Gastric biopsies of the antrum and body and any concerning lesions also should be taken. If no high-risk features are present and the GIM is focal or complete, surveillance is not necessary. H. pylori-associated gastritis, irrespective of sampling and classification, is treated (Fig. 4).
The AGA guidelines emphasize the lack of quality evidence on GIM from the United States. In response, Dr. Setia and colleagues at the University of Chicago are conducting a prospective study to evaluate the risk of gastric cancer among patients with GIM. “I’m hopeful that in the next five years,” she said, “we’ll have meaningful data on intestinal metaplasia follow-up, at least from the Chicagoland area.”
Charna Albert is CAP TODAY associate contributing editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
