Zaibo Li, MD, PhD
Abha Goyal, MD
Eric Huang, MD, PhD
May 2023—Standardized reporting systems have been developed during the past decade for cytopathology of different organ systems including the pancreaticobiliary system. The Papanicolaou Society of Cytopathology (PSC) in 2014 published the first reporting system for pancreaticobiliary cytology.1 Studies have demonstrated that implementation of the PSC reporting system has significantly reduced the number of “atypical” interpretations and increased the number of specific diagnoses.2-8
Recently, the World Health Organization, International Academy of Cytology, and International Agency for Research on Cancer presented a standardized reporting of pancreaticobiliary cytopathology with a seven-tiered system by revising the PSC system for reporting pancreaticobiliary cytology.9 This reporting system is one of the first in a series from various body sites that mirror the WHO Classification of Tumors series and provides an evidence-based terminology system with associated risk of malignancy and diagnostic management recommendation per diagnostic category. The WHO Reporting System for Pancreaticobiliary Cytopathology revises the PSC system and replaces the six-tiered system with a seven-tiered system including “insufficient/inadequate/nondiagnostic,” “benign/negative for malignancy,” “atypical,” “pancreaticobiliary neoplasm-low risk/low grade (PaN-Low),” “pancreaticobiliary neoplasm-high risk/high grade (PaN-High),” “suspicious for malignancy,” and “positive for malignancy” (Table 1).
Insufficient/inadequate/nondiagnostic. A pancreaticobiliary cytology specimen is categorized as insufficient/inadequate/nondiagnostic if it does not permit a diagnosis of the targeted lesion for qualitative and/or quantitative reasons, such as obscuring artifact, gastrointestinal epithelium only, normal elements only in the setting of a clearly defined solid or cystic mass, acellular aspirate of a solid mass, or a cystic mass without evidence of mucinous etiology (i.e. elevated CEA, thick mucin, or presence of KRAS/GNAS mutation). These may be caused by technical, preparation, or sampling issues. There is no minimal number of cells to define this category, but any atypia precludes use of this category.
The average frequency of this category is about 12 percent and the risk of malignancy (ROM) ranges from five percent to 25 percent (higher in bile duct strictures: 28 percent to 69 percent). It is necessary to correlate with clinical information and imaging before providing this interpretation, and it is also important to check the biochemical analysis results in fluids/cyst content for cystic lesions. Repeating aspiration is recommended for patients with this interpretation, and employing rapid onsite evaluation (ROSE) or a different method may be helpful.
Benign/negative for malignancy (non-neoplastic and neoplastic). A pancreaticobiliary cytology specimen is categorized as benign or negative for malignancy if it demonstrates unequivocal benign cytopathological features, which may or may not be diagnostic of a specific process or a benign neoplasm. It should be adequately cellular with no sign of atypia. It may contain only normal pancreatic ductal or acinar cells in the context of a vaguely formed lesion without a distinct mass. Non-neoplastic lesions in this category include benign pancreatic tissue with a vague mass on imaging, inflammatory processes, pseudocyst, lymphoepithelial cyst, and accessory spleen. Benign neoplasms in this category include serous cystadenoma, schwannoma, lymphangioma, and other rare benign neoplasms.
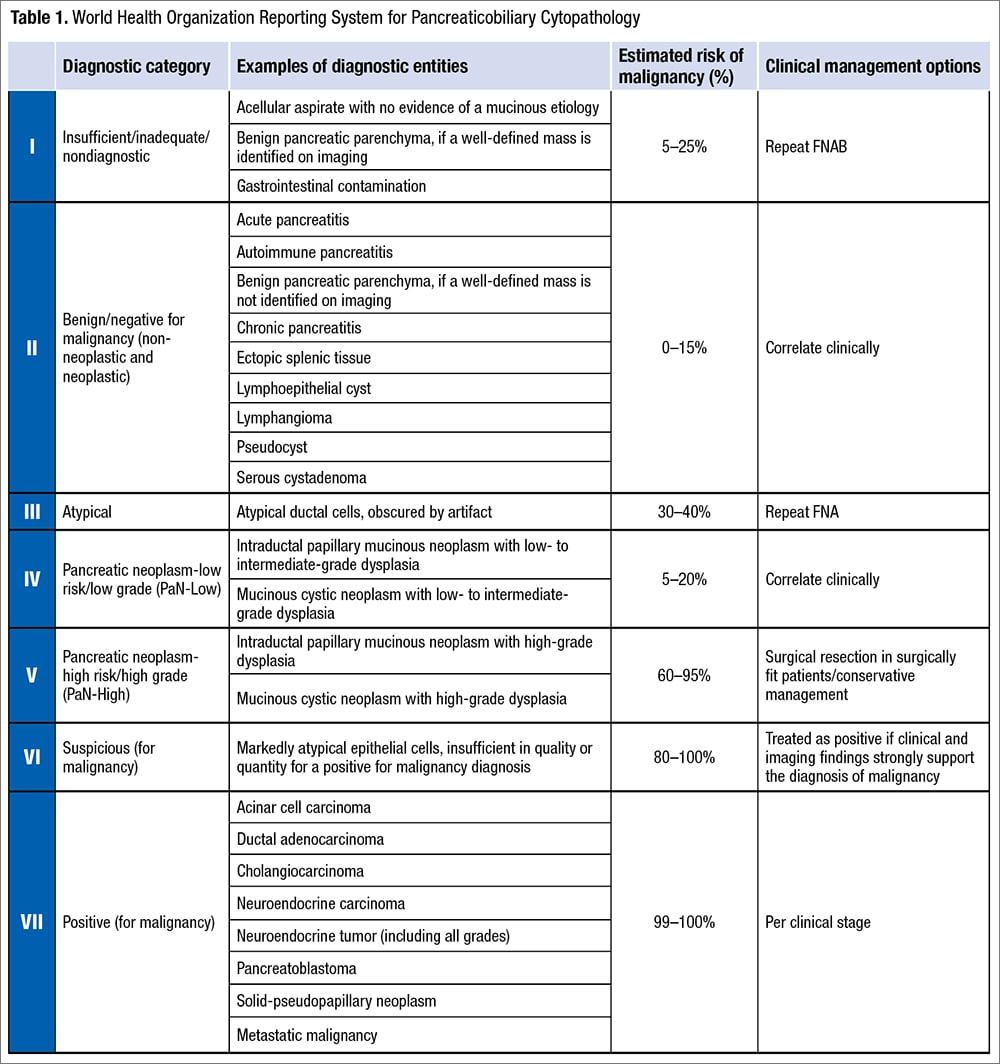
The frequency of this category is about 15 percent and the ROM ranges from zero to 15 percent (higher for bile duct lesions, up to 55 percent). Surgical management usually is not necessary for patients with this interpretation except for large serous cystadenomas.
Atypical. A pancreaticobiliary cytology specimen is categorized as atypical if it demonstrates features that may raise the possibility of a malignant lesion but are insufficient either in number or in quality for the diagnosis of a benign, PaN-Low, PaN-High, or malignant lesion. This interpretation may be due to low cellularity, poor preservation, technical issues, lack of availability of ROSE, or a high threshold for a malignant diagnosis (which is more common with biliary brushings).
The frequency of the atypical category is about 5.5 percent (range: zero percent to 14 percent) in pancreatic fine-needle aspirations. The frequency is higher in bile duct brushings (range: 11 percent to 39.8 percent), resulting from reactive atypia inherent to primary sclerosing cholangitis, stents or biliary stones, and difficulties encountered in the interpretation of well-differentiated adenocarcinomas. ROM of the atypical category is 30 to 40 percent for pancreatic FNAs and 25 to 61 percent for bile duct brushings. Management of patients with atypical interpretation includes consensus review, expert consultation, use of ancillary tests (FISH, molecular testing with next-generation sequencing) to further refine the diagnosis, multidisciplinary discussion, or repeat sampling.
Pancreaticobiliary neoplasm-low risk/low grade (PaN-Low) and high risk/high grade (PaN-High). One of the major changes in the WHO system is the replacement of the original PSC category of “neoplastic: other” by two distinct categories: PaN-Low and PaN-High. This dichotomization was prompted by the inclusion of low-grade and high-grade entities with a widely disparate ROM (14 to 100 percent) together in the PSC system. These two categories of the WHO system comprise the low-grade and high-grade forms of intraductal and/or cystic neoplasms of the pancreaticobiliary tract respectively, i.e. intraductal papillary mucinous neoplasm (IPMN), mucinous cystic neoplasm (MCN), pancreatic intraepithelial neoplasia, biliary intraepithelial neoplasia, and intraductal papillary neoplasm of bile ducts. The high-grade category also includes intraductal oncocytic papillary neoplasm and intraductal tubulopapillary neoplasm.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
