Amy Carpenter Aquino
July 2022—Variability in testing for monoclonal immunoglobulin proteins was in part what led to the new guideline on laboratory detection and initial diagnosis of monoclonal gammopathies, published in May (Keren DF, et al. Arch Pathol Lab Med. 2022;146[5]:575–590).
“Laboratory detection strategies vary considerably for the detection of monoclonal gammopathies. Labs do it in many, many different ways,” said coauthor Mohammad Qasim Ansari, MD, in a CAP21 session last fall intended to spotlight the guideline that had been released online shortly before.
A questionnaire sent in 2016 to 923 laboratories (in 38 countries) enrolled in a CAP protein electrophoresis proficiency test found in the 774 responding labs that 68.6 percent used the agarose gel electrophoresis method and 31.4 percent used capillary zone electrophoresis (Genzen JR, et al. Arch Pathol Lab Med. 2018;142[4]:507–515).
Great variability in testing for monoclonal immunoglobulin proteins (M proteins) was the key finding, said Dr. Ansari, chief of pathology and laboratory medicine, Louis Stokes Cleveland VA Medical Center, and at the time chair of the CAP Diagnostic Immunology Committee.
The most common approach, used by 39 percent of labs, is serum protein electrophoresis (SPE) testing with reflex to immunofixation/immunosubtraction (IFE/ISUB). “And it only reflexes if a monoclonal protein is detected on the initial electrophoresis test. Nineteen percent order SPE only—nothing else,” he said.
Less than 14 percent of labs order both serum protein electrophoresis and IFE/ISUB at the same time, and about 12 percent ask for all four tests (SPE and IFE/ISUB; free light chain; IgG, IgA, IgM). “Amazingly, about 77 percent of orders were for a serum protein electrophoresis with no free light chain assays or urine immunofixation.
“So there is great heterogeneity in the way tests are ordered and how the laboratories perform them,” said Dr. Ansari, who is also professor of pathology, Case Western Reserve University.
In writing the guideline, the expert panel, which Dr. Ansari co-chaired with guideline coauthor David Keren, MD, professor of pathology, University of Michigan, collaborated with members of the American Association for Clinical Chemistry and American Society for Clinical Pathology and included representatives of the American Society of Hematology and International Myeloma Foundation’s International Myeloma Working Group.
“The overarching question that was put to the CAP expert panel was, What are the specimen requirements and what are the appropriate tests needed for the initial laboratory detection of M proteins?” Dr. Ansari said.
The panel recommends, in its first of nine statements, that clinical care providers order serum protein electrophoresis and serum free light chain for the initial detection of M protein in all patients with suspected monoclonal gammopathy.

Dr. Keren
“Our first statement is a strong recommendation,” Dr. Keren said. While most who provided feedback on the statement agreed, “we had several individuals who thought this statement should include serum immunofixation to improve sensitivity,” he said, and others expressed a preference for using urine protein electrophoresis and urine immunofixation over the use of the serum free light chain test.
Panel members chose serum protein electrophoresis because high-quality methods are widely available. “It’s good for the detection of intact monoclonal proteins,” Dr. Keren said, but “unfortunately, even high-quality systems are weak in detecting monoclonal free light chains. These small molecules often pass out into the urine and are not present in large amounts in the serum. That’s why we’ve chosen to add the serum free light chain test.” The International Myeloma Working Group also recommends the use of both tests.
Urine immunofixation is a good test, he noted, but it has not been widely used for decades and is less sensitive than the serum free light chain test, though it does have greater specificity. It’s also more complex for laboratories, which have to perform protein analysis, electrophoresis, and densitometry on the sample.
A study of almost 2,000 patients published in 2009 found that the use of serum protein electrophoresis and the serum free light chain test, as the guideline recommends, detected all cases of multiple myeloma and macroglobulinemia—conditions that require treatment—in that study (Katzmann JA, et al. Clin Chem. 2009;55[8]:1517–1522). For smoldering multiple myeloma (which isn’t treated and for which there is no screening requirement), the two tests detected 99.5 percent of cases. They detected 88.7 percent of monoclonal gammopathy of undetermined significance (MGUS), a condition that progresses at the rate of about one percent a year to multiple myeloma. Due to the inclusion of cases with abnormal free light chain ratios and obvious M-spikes, the cases of MGUS not detected by this screen are at lower risk for progression.
“There is no recommendation to screen for MGUS because there is no treatment for it,” Dr. Keren said. A study is underway in Iceland to see if there is value in screening, he added, “but as of now there’s no recommendation.”
An extrapolation of data from the Katzmann, et al., study indicates laboratories in the CAP study that used only serum protein electrophoresis would miss about 12 percent of cases of multiple myeloma.
The guideline’s second statement says laboratorians should confirm a serum protein electrophoresis abnormality suspicious for the presence of an M protein with additional testing by serum immunofixation electrophoresis or an alternative method with similar sensitivity. “For that, the panel included immunosubtraction or a new way of doing things”—a mass spectrometry method developed at and available from Mayo Clinic, called MASS-FIX, Dr. Keren said, explaining it’s automated and fast, uses the MALDI-TOF instrument, and has replaced immunofixation at Mayo.Dr. Keren shared a study published this year that he coauthored with colleagues at the University of Michigan in which they looked at 1,841 suspicious beta region bands (Kitson AL, et al. Am J Clin Pathol. 2022;157[2]:171–179). “At Michigan, we do an immunofixation or immunosubtraction under three conditions: if the beta region is increased and there is no obvious reason for it such as a liver disease pattern, as a beta 1-2 bridge, or if there’s a suspicious band in the beta region we perform an immunofixation or immunosubtraction.” This identified M proteins in 205 cases (11 percent), similar to the findings of an earlier study (Katzmann JA, et al. Clin Chem. 2010;56[12]:1899–1900).
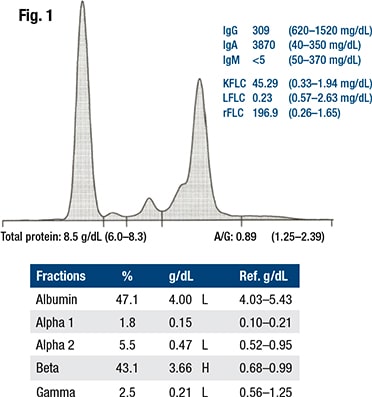 Statement No. 3 in the guideline says laboratorians and/or clinical care providers should follow up an abnormal serum free light chain ratio for the presence of an M protein with serum immunofixation electrophoresis, immunosubtraction, or MASS-FIX.
Statement No. 3 in the guideline says laboratorians and/or clinical care providers should follow up an abnormal serum free light chain ratio for the presence of an M protein with serum immunofixation electrophoresis, immunosubtraction, or MASS-FIX.
The majority of online comments were supportive of this conditional recommendation, Dr. Ansari said, but some suggested removing the serum free light chain testing from the initial investigation. Others suggested performing serum IFE irrespective of the serum free light chain result, “and some suggested adding urine testing to investigate an abnormal ratio of free light chain.”
“With an unexpected abnormal free light chain ratio,” he continued, “when you see it, a serum immunofixation electrophoresis should be used to further investigate the etiology.”
In a study of 18,357 samples at Mayo Clinic, Dispenzieri and colleagues performed serum free light chain assays on all samples, and immunofixation electrophoresis on all samples with an abnormal free light chain ratio or an abnormal protein electrophoresis (Dispenzieri A, et al. Lancet. 2010;375[9727]:1721–1728).
“They found an abnormal ratio of free light chain in 610 cases; 213 of the 610 cases also had IgH expression” and were considered monoclonal gammopathies of undetermined significance, he said.
Of the remaining 397 cases, 146 had one free light chain increased. “This was defined as a new entity—a light chain MGUS,” with a prevalence of 0.8 percent.
Dispenzieri and colleagues also found 57 missed cases of intact M proteins in the initial study. Those cases of MGUS would have been missed if the immunofixation electrophoresis had not been performed, Dr. Ansari said, noting the M proteins were missed on the original serum protein electrophoresis. “So a combination of the three tests was crucial in making the right diagnosis.”
The guideline’s fourth statement says clinical care providers should order serum protein electrophoresis, serum free light chain, serum immunofixation electrophoresis, and urine immunofixation electrophoresis for the initial detection of M protein in all patients with suspected light chain amyloidosis.
AL amyloidosis is an incurable systemic monoclonal gammopathy resulting from deposition of monoclonal free light chains. “Because the quantity of the monoclonal free light chain is small,” Dr. Ansari said, “or its concentration in serum or urine is low due to deposition in the tissue, a combination of several tests is recommended to maximize detection.”
Four percent of the 581 cases of primary amyloid light chain amyloidosis would have been missed in a 2009 Katzmann, et al., study if only the serum protein electrophoresis and free light chain tests had been performed, Dr. Ansari said (Katzmann JA, et al. Clin Chem. 2009;55[8]:1517–1522). If all four of the tests (SPE, FLC, serum IFE, and urine IFE) were done, 98 percent of primary AL cases would have been detected, “which is where we would like to be,” he said.
Palladini, et al. (Palladini G, et al. Clin Chem. 2009;55[3]:499–504), reinforced the need for multiple tests. They looked at cases that were unequivocally identified as AL amyloid with tissue biopsy and immunoelectron microscopy, “so the cases were very well classified,” Dr. Ansari said. And they found that the identification of amyloidogenic light chains cannot rely on a single test and requires the combination of a free light chain assay with immunofixation of both serum and urine.
Two of the guideline’s statements are about what providers should not order, and both are strong recommendations. The first, No. 5, says clinical care providers should not order a heavy/light-chain isotype assay (HLC) for the initial detection of M protein in patients with suspected monoclonal gammopathy.“There is little evidence indicating a use for HLC in the initial diagnosis of monoclonal gammopathies,” Dr. Ansari said. “And lower diagnostic sensitivity is reported when compared with sIFE, sFLC, and SPEP.”

Dr. Ansari
Based on the evidence available (Bradwell AR, et al. Clin Chem. 2009:55[9]:1646–1655; Katzmann JA, et al. Clin Chem. 2015;61[2]:360–367), the panel “believed that the harms of using the assay outweighed any benefit associated with its use,” Dr. Ansari said. The panel also highlighted the risk of missing clinically important conditions like light chain and biclonal gammopathy if a heavy/light-chain isotype assay was used in isolation.
Statement No. 6 says clinical care providers should not use total/intact light chains for the quantitation of M proteins in patients with suspected myeloma. Although the evidence for this recommendation is a single, low-quality study, Dr. Ansari said, “the expert panel proposed a strong recommendation based on a potential for substantial harm to the patient when this assay is used.”
The serum free light chain assay is sensitive and precise, detects much smaller quantities, and is recommended as part of the initial evaluation of patients suspected of having monoclonal gammopathies, he said. “The total light chain assay lacks the same sensitivity and should not be used,” he said, noting it quantifies all antibodies of a particular class, is insensitive, and can miss a small clonal population because of the polyclonal background.
Statement No. 7 in the guideline says in patients with intact M proteins outside the gamma region by serum protein electrophoresis, laboratories should use total immunoglobulin (IgA, IgG, or IgM) for the quantitation of the M proteins. Quantitation of a band in the beta region by serum protein electrophoresis can be performed if the M protein is distinguished from background normal protein bands.“There is relatively little data in the literature to support this statement,” which is a conditional recommendation, Dr. Ansari said. “But it is the consensus of the expert panel.”
Fig. 1 shows an example of a monoclonal protein migrating in the beta region, Dr. Ansari said. “So it overlaps the beta1, which is transferrin, and the beta2, which is the C3 protein. While the M protein needs characterization by IFE or a test of similar specificity, it can be identified as an abnormal band.”
“However, it is difficult to decide where to place the demarcation points to either using the perpendicular drop or the tangent skimming methods, so that you can have an accurate assessment” and can follow that measurement in the future.
The Australasian Association of Clinical Biochemistry and Laboratory Medicine offered guidance for this problem (Tate J, et al. Ann Clin Biochem. 2012;49[Pt 3]:242–256). One of its two suggestions: measure the entire beta region proteins as beta plus paraprotein. “The reasoning is that transferrin and C3 levels are usually relatively constant, so follow-up changes would be reflecting mainly changes in the M protein,” Dr. Ansari said. The problem, he added, is that in the presence of inflammation, transferrin will decline and C3 will increase.
The alternative suggestion was to quantify total IgG, IgA, or IgM in the belief that if broad agreement could be reached, it would harmonize data among laboratories. The AACB group emphasized the importance of using the same method to follow the patient, Dr. Ansari said.
In 2014, Katzmann and colleagues shared the Mayo Clinic recommendation for beta M proteins, which was that if beta M protein is symmetric and less than 2 g/dL, it shouldn’t be measured. Below that level, they felt arbitrary gating of the M-spike would confuse clinicians and patients (Katzmann JA, et al. Clin Chem. 2015;61[2]:360–367). “They offered only IgA to be measured” by nephelometry, Dr. Ansari said, “because this is the most common protein found migrating in this region.”
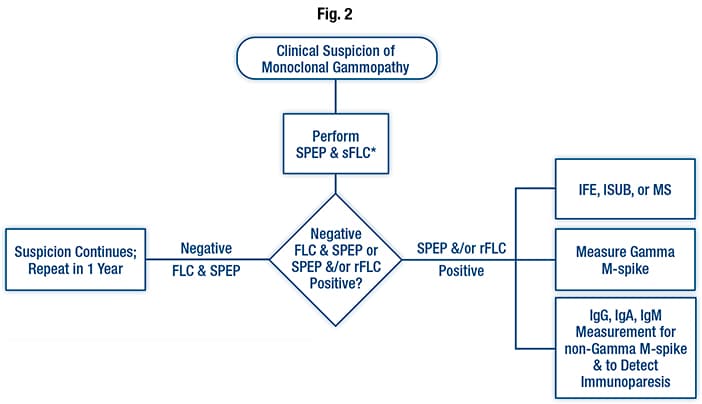
Testing algorithm for monoclonal gammopathies. The asterisk is used to note that when a neuropathy-associated monoclonal process is suspected, a serum immunofixation should be performed. For patients suspected of having amyloid light chain amyloidosis, both serum and urine immunofixation should be performed. Abbreviations: FLC, free light chain; IFE, immunofixation electrophoresis; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; ISUB, immunosubtraction; MS, mass spectrophotometry; M-spike, monoclonal spike; rFLC, free light chain ratio; sFLC, serum free light chain; SPEP, serum protein electrophoresis. Reprinted from Keren DF, Bocsi G, Billman BL, et al. Laboratory Detection and Initial Diagnosis of Monoclonal Gammopathies: Guideline from the College of American Pathologists in Collaboration with the American Association for Clinical Chemistry and the American Society for Clinical Pathology. Arch Pathol Lab Med. 2022;146(5): 575–590 with permission from Archives of Pathology & Laboratory Medicine. Copyright 2022. College of American Pathologists.
Also in 2014, the International Myeloma Working Group, “recognizing the difficulties in obtaining a consistent measurement for beta-migrating myopathies, recommended adopting the suggestions of both groups,” Dr. Ansari said. “However, they were not as eager to use the IgM or IgG because they felt that they were not as accurate.” Although IgA monoclonal proteins predominate in the beta region, IgG and IgM may be present too, Dr. Ansari said, as well as occasional cases of monoclonal free light chains. “Consequently, the CAP expert panel chose to add these options when the M protein cannot be distinguished from other proteins. So we are allowing IgG, IgA, and IgM to be measured by nephelometry in those cases.”
The use of immunosubtraction does allow the laboratory to distinguish the M protein from other proteins such as transferrin and C3, thereby allowing the laboratory to provide a measurement. “However, such measurements are difficult to harmonize between laboratories, which is why use of the total immunoglobulin measurement may be preferable,” Dr. Keren says.
The guideline’s eighth statement says laboratorians should report both quantitative levels of free kappa and free lambda and the free light chain ratio when the serum free light chain assay is performed.
A study by Rajkumar and colleagues (Rajkumar SV, et al. Blood. 2005;106[3]:812–817) identified three important risk factors for MGUS progression to multiple myeloma: an M protein spike of 1.5 g/dL or greater, an abnormal ratio of the serum free light chains, and having a non-IgG M protein. One risk factor would mean normal progression risk, which is about 20 percent; no risk factors only five percent; and patients with all three risk factors “progressed at almost 60 percent—three times the average case of MGUS,” Dr. Keren said. “So it is very important to include this ratio of free light chains in determination.”
Laboratory measurements of the percentage of bone marrow plasma cells and serum free light chain levels detailed in a 2013 risk evaluation of smoldering multiple myeloma were key to reclassifying the high-risk patients as having treatable multiple myeloma (Dispenzieri A, et al. Blood. 2013;122[26]:4172–4181).
They reported that two to three percent of patients with greater than 60 percent bone marrow plasma cells had active multiple myeloma and required treatment, Dr. Keren said. “That is why the most recent International Myeloma Working Group classification of multiple myeloma includes cases with bone marrow plasma cells greater than 60 percent, irrespective of whether an intact M protein or abnormal free light chain can be detected.” Further, 15 percent of patients formerly classified as smoldering multiple myeloma having a ratio of involved free light chain to uninvolved free light chain of 100 or greater were also reclassified as multiple myeloma, allowing earlier treatment.
The final statement says clinical care providers may use rFLC, IgM isotype, M protein greater than 1.5 g/dL, and immunoparesis as risk factors for progression to multiple myeloma or a B-cell lymphoproliferative disorder.
This statement duplicates parts of other statements, Dr. Keren said, “but this puts them in a group of risk factors for progression and includes another new factor”—increased risk if there was a decrease in the noninvolved isotypes below reference intervals. “That is, if you have an IgG multiple myeloma or monoclonal protein, and if the IgM and/or the IgA are decreased, that is called immunoparesis, and that would be an increased risk for patients moving on toward multiple myeloma or other B-cell proliferative disorders.”
In Fig. 2 is the guideline’s testing algorithm for monoclonal gammopathies. If there is a clinical suspicion of monoclonal gammopathy, an SPEP and FLC should be performed, at a minimum, Dr. Keren said. “However, you need to add serum immunofixation for all patients with neuropathies related to monoclonal gammopathies such as POEMS syndrome because their M proteins may be too small to be seen without immunofixation.”
For patients with AL amyloid, “we recommend adding both serum and urine immunofixation to the protein electrophoresis and the free light chain to optimize laboratory detection.”
A negative result in patients with continued suspicion of harboring a monoclonal gammopathy warrants a repeat test in one year, while a positive result for the serum protein electrophoresis and/or free light chain requires either immunofixation, immunosubtraction, or mass spectrometry for identification.
Amy Carpenter Aquino is CAP TODAY senior editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
