Charna Albert
July 2022—Good slides in, good images out.
Liron Pantanowitz, MD, MHA, explained how to get those good images in a recent webinar on preanalytics quality control in digital pathology, sponsored by Sunquest and made available by the Association for Pathology Informatics.
“Most of the time when people talk about digital pathology and work on digital pathology, including the applications, they don’t pay too much attention to the preimaging component,” said Dr. Pantanowitz, professor of pathology and director of the Division of Anatomic Pathology, University of Michigan Health. “But if you have poor slides and poor tissue, how can you expect to have a good whole slide image and ask a pathologist to make a primary diagnosis on that image when you haven’t gotten the first part right?”
While a perfect slide and perfect image are the hope, he said, imperfect slides and images shouldn’t impair the ability to make a diagnosis. “Minor artifacts we can brush aside, but major artifacts we should not.”
Whole slide imaging of breast pathology is particularly affected by preimaging factors, he said, citing a study from pathologists at Nottingham University Hospitals. The authors examined 40,160 whole slide images of breast core biopsies and resection specimens and compared them with the corresponding glass slides, most of which were H&E slides (Atallah NM, et al. Mod Pathol. Published online Dec. 27, 2021. doi:10.1038/s41379-021-01000-8). “The main problem they reported—which I’ve heard from colleagues around the U.S.—was that with breast cases you can end up with pieces of tissue missing in your digital image,” Dr. Pantanowitz said. It happened mostly on the periphery, near the edge of the slides, and “one of the main culprits,” he said, was the fatty tissue, which is pale and thus can be missed by tissue detection algorithms. Atallah, et al., reported missing tissue in the images of the breast resections at a frequency of two to 19 percent (no missing tissue was identified in the core biopsy specimens). Though the area size of the missing tissue ranged from one to 70 percent, in more than 75 percent of cases it was less than 10 percent and peripherally located on the slide, making it more likely to be cropped from scanning regions. In all cases, the missed tissue was fat with or without small entrapped normal breast parenchyma.
Fig. 1, from the study, is an overview image of a fully scanned glass slide (A) and a high-power (40×) image of a normal breast terminal lobular duct unit (1.5 mm at largest diameter) present at the periphery of the slide (B) and missed on scanning (C).
“Who wants a digital pathology setup where close to 20 percent of the breast slides cannot be digitized or you’re going to be missing key tissue? No one would want to rely on that and be held liable for that kind of a problem,” Dr. Pantanowitz said.
The authors used manual quality control measures that involved macroscopically evaluating all slides for preanalytic artifacts before scanning. The scanner performed additional real-time QC, displaying user interface messages when barcode detection failures, macro focus image failures, and image quality errors occurred, and when no tissue was detected. Post-scan QC consisted of a review of image thumbnails (the low-resolution entire slide view of the whole slide image) to ensure all tissue on the glass slide was scanned. These protocols reduced WSI failure rates sevenfold, but checking every slide manually “required a huge investment of time upfront,” Dr. Pantanowitz said.
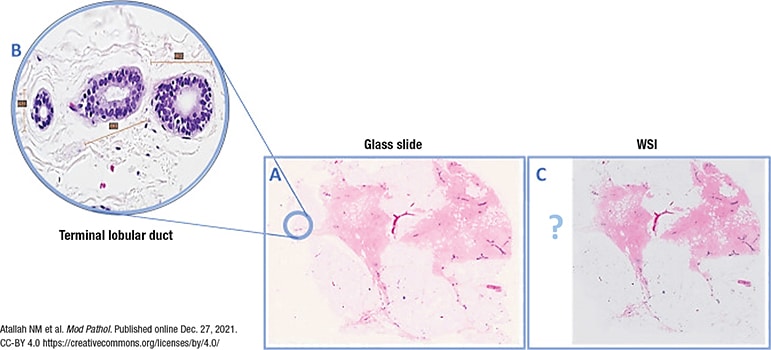
Fig. 1. WSI missing a normal breast duct at the slide periphery
(A) Overview image of a slide that was fully scanned. (B) High power of the normal breast terminal lobular duct unit on the glass slide (×40 light microscope) present at periphery of slide and missed upon scanning (C).
The CAP’s updated whole slide imaging validation guidelines were published in April (Evans AJ, et al. Arch Pathol Lab Med. 2022;146[4]:440–450). Good practice statement No. 7 says the validation process should confirm all material present on the glass slide is included in the digital image. Laboratories can do this by checking the digital image against the paraffin block, if it’s available, checking the glass slide (though that adds a redundant step), or checking the macro-image the scanner takes of the entire slide, which includes the label, barcode, and all fragments of tissue, Dr. Pantanowitz said. The macro-image, he noted, is different from the thumbnail. “The thumbnail will not tell you if there’s tissue missing—it only tells you what tissue got scanned.” Many systems do not display the macro-image, however. “It requires a few more steps to find the macro-image, unless you work with your vendor to readily display it.”
BlocDoc is a digital device that offers pathology labs a way to satisfy good practice statement No. 7 (Dr. Pantanowitz is a BlocDoc co-inventor). Many pathology labs today are geographically separated from their hospitals and thus the paraffin blocks. With BlocDoc, the blocks can be examined remotely. The device takes a digital image of the cut surface of the paraffin block, which is then uploaded into the laboratory information system and attached to the appropriate case based on the cassette barcode. Using BlocDoc, pathologists can verify whether all tissue from the block has been scanned. It also permits a polarized view of the block, making it easier to visualize tissue embedded deep within the block.
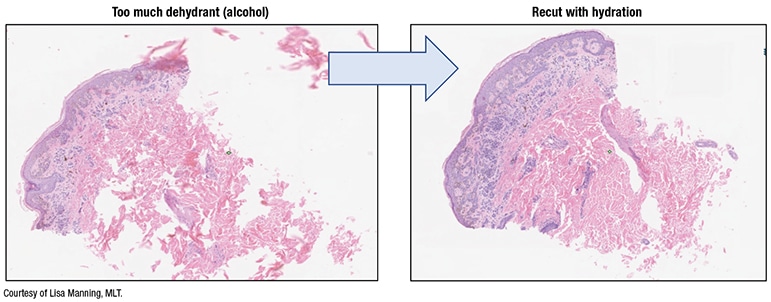
Fig. 2. Tissue processing impact
In a study published in the Journal of Pathology Informatics, L’Imperio, et al., reported fewer discrepancies with fragmented tissue specimens—transurethral resections of prostates, nasal polypectomies, and piecemeal uterine myomectomies, for example—when using BlocDoc in their digital workflow (L’Imperio V, et al. J Pathol Inform. 2021;12:32). “With all those fragments of tissue,” Dr. Pantanowitz said, “you’re never quite sure if all the fragments made it onto the slide from the block and all got scanned.”
Preimaging artifacts can make the pathologist’s job more difficult, Dr. Pantanowitz said. One example: reading frozen sections via telepathology when there are tissue folds, “especially if it was scanned in one plane and you don’t have access to focus up and down.” And slide artifacts can wreak havoc with biomarker image analysis algorithms, “and who knows what they will do with AI.” One study using the Visiopharm HER2 image algorithm demonstrated, for example, that blurred slides, as can occur when there is excess mounting medium on the slide coverslip, affected HER2 testing scores, turning a 1+, 2+, or 3+ into a 0 score (Pantanowitz L, et al. J Pathol Inform. 2017;8:39). “So you can significantly alter a major diagnosis for a patient, therefore incorrectly putting them into a therapy pathway, just based on too much mounting medium on the slide.”“Be mindful,” Dr. Pantanowitz said, “that every slide that gets made in your histology lab will eventually be digitized.” His advice:
- Place specimens into fixative as soon as possible. “When I participated for 10 years in international telepathology consultations at the University of Pittsburgh Medical Center, poorly fixed tissues coming from rural areas outside the U.S. were almost impossible to examine on a whole slide image. So you want quality formalin fixation,” he said.
Open specimens whenever possible with thin slices to maximize formalin exposure, and use high-quality fresh neutral buffered formalin for the appropriate time. The ideal ratio of formalin to tissue: 15–20:1. And fatty specimens may require longer processing schedules than biopsies and small specimens. - In the grossing step, “don’t fill the cassettes with huge pieces of tissue that go all the way to the edge,” he said, because if they do, they’re also likely to reach the edge of the glass slide, where they cannot be incorporated into the bounding box and so will be out of focus or not scanned. Tissue should be no thicker than 4 mm—about the size of a quarter—and trimmed to fit easily within cassettes. And the number of tissue pieces per cassette should be limited.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
