In our case, ischemia, infection, and recurrent lymphoma were high on the list of differential diagnoses. Although a single stool sample was negative for Clostridium difficile and Norovirus, specimen limitations precluded additional studies. In the absence of specific findings, the patient was maintained on broad-spectrum antibiotics and prophylaxis with fluconazole. Azoles generally lack activity against Zygomycetes.1 Amphotericin B is the agent of choice for most Mucorales, although its efficacy is variable depending on the specific organism.1 A second case of zygomycosis occurred in our institution, also requiring molecular identification, and was shown to be the same species as in this case. However, because of the lack of a pure culture specimen, further testing for infection control studies proved to be challenging. A recent study describing an outbreak of gastrointestinal zygomycosis due to Rhizopus microsporus had patients who presented with segmental bowel thickening and intra-abdominal abscess formation; however, the spectrum of clinical manifestation ranged from asymptomatic mucosal colonization to invasive disease.4
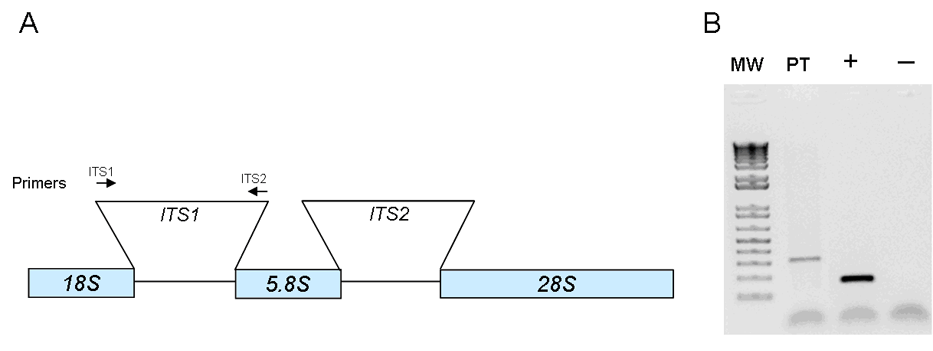
Fig. 4. A) Fungal ribosomal DNA repeat unit showing coding regions (18S, 5.8S, and 28S) and internal transcribed regions (ITS1 and ITS2). B) Agarose gel showing the ITS1 PCR product (PT) obtained from DNA extracted from PET A7 (abscess behind stomach). The first column on the gel is the molecular weight marker (MW). The patient sample (PT) is analyzed simultaneously with the positive (+) and negative (-) controls for comparison.
Diagnosing zygomycosis is difficult because tissue culture is often negative.5 Although the gold standard for diagnosis is histologic examination, fungal morphology, even aided by special stains (PAS and GMS), is unreliable5 for use as the only means of identification. There is a chance for incorrect diagnosis if adequate fungal hypha or the classic morphology is not seen. In addition, microscopy cannot differentiate fungi beyond the genera level. Fluorescent antibody staining and newer immunohistochemical methods have shown improved identification over conventional modalities (H&E and special stains); however, these methods are not yet widely used.5 Culture-independent nucleic-acid based methods such as PCR are rapid and sensitive and can be used on fresh or formalin-fixed tissue. Targeting of multi-copy loci, particularly the ribosomal DNA genes (18S, 28S, and 5.8S) and the intervening internal transcribed spacer (ITS) regions (ITS1 and ITS2), allow increased specificity with identification to the species level.6 Such specificity is important in selecting appropriate antifungal therapies.
With the high mortality rates of invasive fungal infections, the autopsy becomes an important tool to study the patterns and prevalence of such infections. It provides key information for hospital QA/QC and has the potential to identify emerging infections. In addition, unexpected diagnoses from autopsy provide valuable learning experiences, such as the need to maintain a high degree of suspicion for zygomycosis in vulnerable patient populations, as exemplified in this case. Molecular microbiology techniques have proved to be a significant source of data for these purposes. The information gained will better equip clinicians to manage these devastating infections.
Conclusion
This case emphasizes the importance of including zygomycosis in the differential diagnosis, especially in immunocompromised patients with unusual presentations and multiple risk factors. As fungal culture can be insensitive and morphology can be unclear, molecular techniques are critical tools in the diagnosis and selection of therapy for these cases. Using such techniques in the postmortem setting can aid in infection control and in understanding disease epidemiology, with the potential for improving outcomes in future patients.
References
- Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of clinical manifestations, diagnosis, and treatment. Clin Microbiol Infect. 2004;10(suppl 1):31–47.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):s23–s34.
- Harrington AT, Creutzfeldt CJ, SenGupta DJ, et al. Diagnosis of neurocysticercosis by detection of Taenia solium DNA using a global DNA screening platform. Clin Infect Dis. 2009;48:86–90.
- Cheng VCC, Chan JFW, Ngan AHY, et al. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009;47(9):2834–2843.
- Sangoi AR, Rogers WM, Longacre TA, et al. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol. 2009;131:364–375.
- Munoz-Cadavid C, Rudd S, Zaki SR, et al. Improving molecular detection of fungal DNA in formalin-fixed tissues: comparison of five tissue DNA extraction methods using panfungal PCR. J Clin Microbiol. 2010;48(6):2147–2153.
Dr. Marshall is a PGY3 resident in anatomic pathology, Dr. SenGupta is a research scientist in laboratory medicine, Dan Hoogestraat is a laboratory technologist in laboratory medicine, Dr. Stephens is a professor of laboratory medicine, Dr. Cookson is a professor of laboratory medicine, and Dr. Yeung is an assistant member at Fred Hutchinson Cancer Research Center and an acting assistant professor in anatomic pathology—all at the University of Washington, Seattle.
Test yourself.
Here are a few take-home points and questions. Answers to the questions are online now at www.amp.org/casereviews and will be published in CAP TODAY next month.
1. Zygomycoses are important to recognize and treat aggressively in immunocompromised patients due to a highly morbid and often fatal clinical course.
Which statement is false?
a) Zygomycosis may cause a rapidly fatal, clinically obscure infection even in non-immunocompromised patients.
b) As a class of antifungals, azoles have good efficacy against Zygomycetes.
c) The stomach is the most common site of infection in gastrointestinal zygomycoses.
d) Zygomycoses may infect a wide range of tissue sites, possibly leading to uncommon presentations that evade early detection.
2. In tissue, zygomycosis may show variation from normal characteristic morphology (variable-width ribbon-like hyphae, irregular branching, aseptate), but are typically PAS positive and silver negative.
Classic Zygomycete morphology includes:
a) 45-degree branching.
b) Narrow filamentous hyphae.
c) Budding yeast forms.
d) Fruiting bodies.
e) No to weak reactivity with silver special stains.
3. Molecular identification of organisms in paraffin-embedded tissues is a rapid and accurate diagnostic test, and if done in a timely manner, can guide clinical therapies.
Which diagnostic modality cannot identify fungal organisms beyond the genera level?
a) Fungal culture.
b) Ribosomal DNA sequencing.
c) Histomorphology and special stains.
d) MALDI-TOF mass spectrometry.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
