Individuals who identify as having an egg yolk allergy are often found to have a kind of asthma due to inhalation of bird dander. “It’s not perfect,” he said, but “if you use the available tests—both specific and otherwise—you can generate a clinical picture.”
For component test reporting at Mayo Clinic, “we report down to 0.10 kU/L,” Dr. Bornhorst said (Fig. 1).
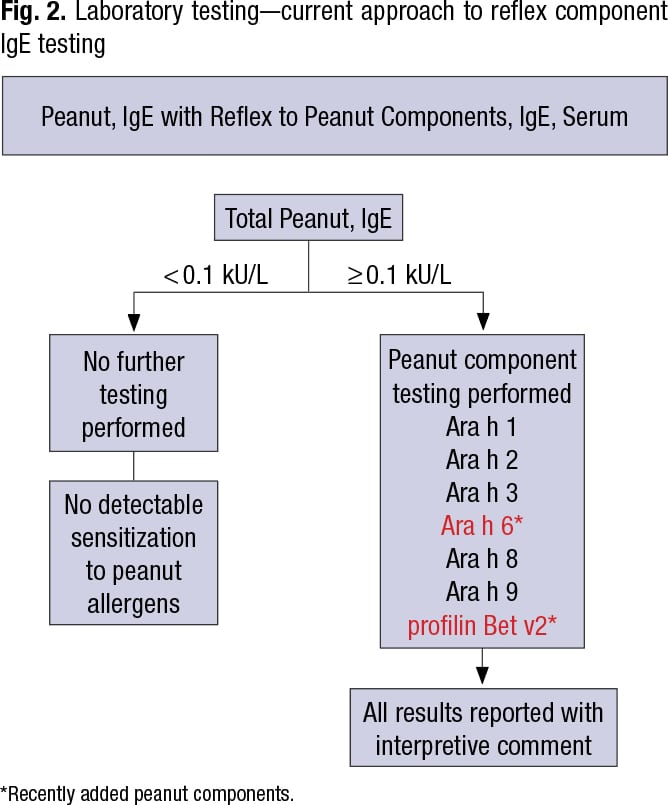
For peanut component antibodies, Mayo Clinic traditionally has tested for five main components—Ara h 1, 2, 3, 8, and 9—which are the most allergenic of the 30 peanut proteins, 18 of which are associated with sensitization, he said. However, the major component proteins account for about 90 to 95 percent of peanut allergy.
Mayo Clinic recently added two peanut components—Ara h 6 and profilin Bet v2—to its menu. “Profilin Bet v2 is a more cross-reactive protein,” Dr. Bornhorst said in an interview, “and the other, Ara h 6, is similar to a preexisting protein component [Ara h 2] within the previous five-component panel. The addition of more complements is intended to improve the sensitivity of peanut component testing relative to the overall specific total peanut IgE extract assay.”
“So you have a mixture of proteins, you do your specific total peanut test. If it’s positive you reflex to component testing, and then you report that with an interpretive comment.” It’s a mixture of specific at the start and component testing to generate a clinical picture (Fig. 2).
One reason for this, he said, is that different components have different resistance to digestion and heat and different amounts of severity, which can guide patient allergen avoidance or treatment. Ara h 2 is often considered to be the most severe allergen in terms of allergic response severity.
Oral allergy syndrome is a type of food allergy in which allergic reaction occurs in the mouth after eating certain fruits, nuts, or vegetables, including peanuts, he said. It’s associated with cross-reactivity of Ara h 8 (a Bet v1 homologue that is cross-reactive with birch pollen allergen) and Ara h 9 (a nonspecific lipid transfer protein that is cross-reactive with pitted fruit allergen). Thus, component testing can be used to differentiate different types of potential allergenic response.
A comparative study of diagnostic test characteristics of the peanut skin prick test, peanut-specific IgE serum antibody testing, and IgE antibody testing for peanut components, in a screening population of 321 infants before known peanut exposure, revealed that Ara h 2 is the most specific allergen, Dr. Bornhorst said (Keet C, et al. J Allergy Clin Immunol. 2021;147[3]:977–983.e2). At a cutoff point of 0.1 kUa/L, Ara h 2-sIgE discriminated between allergic and nonallergic best. Total peanut IgE was found to be the most sensitive but not nearly as specific. “So there are advantages of considering using Ara h 2 as a frontline test, in some populations, or as is done in Mayo peanut component reflex panel testing—testing for total specific IgE first, and then reflexing to subsequent component testing to increase overall sensitivity for allergic reaction,” he said.
Because the specificity of “specific” assays is not 100 percent, Dr. Bornhorst advises against the routine use of large screening panels or annual testing on an individual without clinical indication of allergy. “You will get false-positives.”

Dr. Bornhorst
In addition to its launch of Ara h 6 and profilin Bet v2, Mayo Clinic recently launched its bromelain MUXF3 testing to evaluate for the presence of antibodies to cross-reactive carbohydrate determinants. CCD structures are found in plant and insect glycoproteins and are commonly recognized by IgE antibodies as epitopes that can lead to widespread cross-reactivity to different allergens and potentially cause false-positives in in vitro diagnostic serum IgE antibody testing results (Sinson E, et al. PLoS One. 2020;15[4]:e0231344). “There’s increasing interest in some platforms as to what degree of cross-reactivity or interference you may see in positive serum IgE antibody results due to these cross-reactive determinants. So there’s interest in designing assays away from that and then also including such things as cross-reactive inhibitors and blocking agents,” Dr. Bornhorst tells CAP TODAY.
IgE antibodies against bromelain is a test for this cross-reactivity since its MUXF3 carbohydrate chain is found in many plant proteins, including peanuts. Sinson, et al., analyzed sera from patients sensitized to peanut, silver birch, and/or timothy grass against the MUXF3 serum IgE, and as much as 35 percent of the sensitized group tested in the study was found to be reactive to CCD allergen (≥0.35 kU/L), Dr. Bornhorst said.
“There is some growing evidence that CCDs cross-react and cause multiple false-positives in serum IgE antibody testing, and it may be dependent in an instrument platform- and assay/allergen-specific manner,” he said. The interest in CCD inhibitors, blocking agents, and assay markers of CCD interference is growing, he said, but CCDs generally don’t cause clinical allergic reaction.
Miguel Park, MD, allergist-immunologist, internist, and assistant professor at Mayo Clinic, said in the AACC session, in which he was a co-presenter, that the history of the reaction is one of the drivers of whether to test, and risk factors and allergen prevalence, too, factor into the pretest probability. Then it’s a question of posttest probability: “Will the skin test or IgE serum testing, whether it’s component or whole or even the oral challenge, further our pretest probability to a higher probability?”Dr. Park reported on the case of a 13-month-old male patient who was seen for a possible peanut allergy. Clinically (and with repeated exposures), the pretest probability that the patient had a peanut allergy was high. Dr. Park ordered a blood test for the patient, and the result was an IgE of 7.65 kU/L (in the positive range). How to interpret these tests is the question, he said. If the pretest probability is high and the skin test or serum IgE test is positive, then it helps confirm the diagnosis. However, if the pretest probability is low and the skin test or serum IgE test is positive, then the testing may not help in the diagnosis. Positive skin prick and serum IgE tests may indicate sensitization but do not alone equate to clinical diagnosis. “As clinicians, we think of these tests as having high sensitivity but low specificity.”
Cutoffs have been developed for certain foods, and while they’re “wonderful to have,” Dr. Park said, caution is needed because they were established in tertiary medical center studies in patients with moderate to severe atopic conditions (e.g. severe atopic dermatitis) and with extensive family history. “It makes you wonder about their generalizability and whether they’re relevant to your patient population.”
Some studies have suggested that component testing has high specificity and positive predictive value and could make it unnecessary to do an oral challenge, Dr. Park said. It may have an advantage in situations in which the patient is sensitized without a history of clinical reaction, or when skin and serum tests are discrepant. “And possibly it might help predict the severity of the reactions in the future,” he said. It also has much better specificity than skin testing or specific IgE testing to particular foods (Anagnostou A. Ann Allergy Asthma Immunol. 2019;122[6]:576–579).
The limitations of component testing can be seen in the variabilities in cutoffs (Flores Kim J, et al. Allergy. 2018;73[8]:1609–1621), sensitivity, specificity, and positive and negative predictive values. “It kind of makes you argue that maybe we’re not ready for prime time with these tests,” Dr. Park said.
Professional societies have been trying to find a way to merge the clinical history with component testing, he said, and one group advocated for using likelihood ratios to go from pretest to posttest probability (Greenhawt M, et al. J Allergy Clin Immunol. 2020;146[6]:1302–1334). “It’s not dependent on population prevalence of disease, and they think it’s more adaptable to individual clinical settings,” he said.
Greenhawt, et al., wrote that diagnostic testing (skin prick or serum IgE) should not be used in patients in whom there is low or very low pretest probability of peanut allergy. “The bottom line,” Dr. Park said, “is that if a patient has a high pretest probability, they’re giving us clinicians the go-ahead to use any of them—skin, blood, Ara h 2 component tests” within the Fagan nomogram. But they caution against the routine use of component testing in addition to either skin testing or serum-specific IgE to whole peanut to increase diagnostic accuracy.
Amy Carpenter Aquino is CAP TODAY senior editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
