Editor: Frederick L. Kiechle, MD, PhD
Submit your pathology-related question for reply by appropriate medical consultants. CAP TODAY will make every effort to answer all relevant questions. However, those questions that are not of general interest may not receive a reply. For your question to be considered, you must include your name and address; this information will be omitted if your question is published in CAP TODAY.
“Q&A” is devoted this month to a question about HER2 testing in colorectal cancer.
Q. I am a community pathologist and would like to know if the CAP has recommendations on diagnostic criteria for evaluating HER2 in colorectal carcinoma. There appears to be more than one set of criteria in various references (i.e. HERACLES, Ventana), and when clinicians request the test, I am not sure how best to evaluate these specimens.
A. February 2020—The CAP has no official position on HER2 testing in colorectal cancer. The following represents expert opinion based on my experience as an immunohistochemistry laboratory director and longtime member of the CAP Immunohistochemistry Committee and my careful review of the literature. This response was peer reviewed by other GI pathologists on the Immunohistochemistry Committee. I will discuss the HERACLES and so-called Ventana diagnostic criteria and other aspects germane to HER2 testing in non-breast/non-gastroesophageal carcinomas. Of note, anti-HER2 therapy is only FDA approved in breast and gastric/gastroesophageal junction adenocarcinomas, and treatment of other HER2-positive carcinomas should be considered only in patients who have failed conventional therapies and/or in the context of a clinical trial.Two recent phase two clinical trials demonstrated moderate activity of dual anti-HER2 therapy in advanced colorectal cancer. The HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) trial enrolled 27 KRAS wild-type, HER2-positive patients for treatment with combined trastuzumab (a monoclonal antibody to extracellular subdomain IV of HER2) and lapatinib (a small-molecule inhibitor of HER2 and EGFR). Eight (30 percent) patients achieved an objective response, including one complete response and seven partial responses; 12 additional patients had stable disease.1 HER2 positivity was defined by the HERACLES Diagnostic Criteria, formulated specifically to select patients for this clinical trial; testing was performed centrally.2
MyPathway is a phase two multiple basket trial, enrolling patients with diverse solid tumor types for targeted anti-HER2, BRAF, EGFR, or Hedgehog signaling pathway therapy. Investigators reported on 57 HER2-positive advanced colorectal cancer patients, enrolled regardless of KRAS status, treated with combined trastuzumab and pertuzumab (a monoclonal antibody to extracellular subdomain II of HER2, which inhibits HER2/HER3 dimerization). Eighteen (32 percent) patients achieved an objective response, including one complete response and 17 partial responses; seven additional patients had stable disease for greater than four months.3 HER2 positivity was defined as any of the following: 1) HER2 IHC 3+ in >10 percent of cells, 2) HER2:CEP17 ratio ≥2.0 or HER2 count >6 per cell, 3) increased HER2 gene copy number by molecular methods, and/or 4) HER2 activating mutations, including exon 20 insertions; deletions around amino acids 755–759; G309A, G309E, S310F, D769H, D769Y, V777L, P780-Y781insGSP, V842I, R896C; or previously reported activating mutations (or indels) in COSMIC. HER2 testing was performed by local CLIA-certified laboratories.
Pathologists have expressed confusion about which HER2 testing criteria to apply in colon cancer. I have also heard pathologists say they have been told not to use gastric/gastroesophageal junction criteria. The HERACLES criteria were designed to select patients for a clinical trial. Implicit here is the goal to have a positive clinical trial. A (unprecedented) 50 percent threshold for IHC positivity was established in a cohort of only 17 positive cases.2 The 50 percent threshold optimized the accuracy of positive Ventana Pathway (FDA-approved kit) immunohistochemistry to predict ISH positivity, which was also set at an unprecedented 50 percent cellularity threshold. These same cases were also examined using the Dako A0485 polyclonal antibody with immunohistochemistry performed manually. Using this assay in the same set of cases, a 10 percent positive threshold was most accurate. Nevertheless, the authors decided to implement a 50 percent threshold for the clinical trial.
For the purpose of identifying the most patients who would potentially benefit from a therapy, I found these criteria to be overly restrictive. I was also concerned that the results of testing generated by a central laboratory would not necessarily be generalizable. After the ToGA trial in gastric/gastroesophageal junction cancers, there was opportunity to further evaluate the interlaboratory and interobserver reproducibility of trial-derived HER2 testing criteria.4 All that said, before the publication of the positive MyPathway trial, these were the only criteria that were clinically validated.
The only reference I could find to Ventana colorectal cancer HER2 testing criteria is in an excellent review of the state of the bench-to-bedside science of HER2 in colon cancer,5 coauthored by many of the authors of the HERACLES clinical trial.1 In the table in which they list the Ventana criteria, they reference a review article on HER2 testing in gastric cancer6 by some of the same authors of the HER2 testing guidelines developed in the setting of the ToGA trial7—that is to say, the Ventana colorectal cancer HER2 criteria are equivalent to those used in resections of gastroesophageal adenocarcinomas (which may be variously referred to as ToGA or Ruschoff/Hofmann criteria and which were adopted in the CAP/ASCP/ASCO gastroesophageal adenocarcinoma HER2 guideline).8 The MyPathway trial used these latter, less restrictive criteria, along with a couple of novel molecular ones. I use these criteria in my practice.

Table 1 compares the CAP/ASCP/ASCO gastroesophageal and HERACLES colon HER2 immunohistochemistry criteria. In bold are the areas where the interpretation or subsequent testing/clinical consequences differ. In many cases, these are the same. Both sets of criteria recognize lateral membrane and basolateral (U shaped) staining as potentially positive staining patterns. The HERACLES criteria assign patients with weak to moderate specific staining in 10 to 49 percent of tumor cells to the negative category; according to CAP/ASCP/ASCO criteria, these tumors should be tested by in situ hybridization (ISH). The HERACLES criteria dictate repeat IHC in tumors with 2+ staining in ≥50 percent of tumor cells, while by CAP/ASCP/ASCO criteria, one would proceed directly to ISH. Similarly, the HERACLES criteria specify repeat IHC in tumors with 3+ staining in 10 to 50 percent of tumor cells, as well as confirmatory ISH, while by CAP/ASCP/ASCO criteria, these would be considered positive.
My main concern with the HERACLES IHC criteria is that they deny patients with IHC 2+ staining in 10 to 49 percent of cells the opportunity to be positive by ISH. In the initial HERACLES validation study, one of the 17 ISH-positive tumors showed 2+ staining in 20 percent of cells using the A0485 antibody.2 These criteria also dictate repeat IHC testing in scenarios in which one might otherwise proceed directly to ISH or consider a case positive, as well as additional ISH in one scenario (i.e. 3+, ≥10 percent) otherwise typically considered positive by IHC alone.
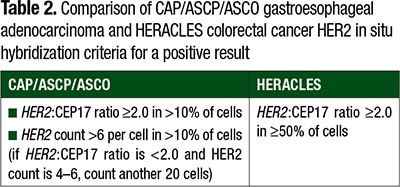
Table 2 compares the CAP/ASCP/ASCO gastroesophageal and HERACLES ISH criteria. The HERACLES criteria again set a positivity threshold as amplification in ≥50 percent of tumor, while the CAP/ASCP/ASCO threshold considers amplification in >10 percent and allows for positivity in instances in which the HER2 count is >6 per cell, even in the absence of a HER2:CEP17 ratio ≥2.0 (a scenario that may occur due to co-amplification of HER2 and CEP17). These latter, more permissive criteria were used in the MyPathway trial.
I was interested in the impact that increasing the threshold for IHC positivity from 10 to 50 percent might have on clinical testing. This depends, of course, on the frequency of this extent of staining in a given tumor type, which reflects a tumor’s tendency for heterogeneity of HER2 overexpression. In breast cancer, HER2 expression tends to be uniform. Thus, changing the threshold for positivity from 30 percent to 10 percent (per ASCO/CAP 2007 and 2013 guidelines, respectively) has had little effect on rates of IHC 3+ (ranging from increases of 0.5 to 1.5 percent in the three studies I could find that applied 2007 and 2013 IHC criteria to the same cohorts).9-11 Gastroesophageal adenocarcinomas are inherently more heterogeneous, with up to 30 percent of HER2-amplified cases having <30 percent cells staining by IHC.6 In the HERACLES Diagnostic Criteria validation study, two (with A0485) and one (with Pathway) of 17 ISH-amplified cases showed 3+ staining in 10 to 30 percent of cells.2 In another recent study by Shimada, et al., among 201 colorectal cancers, 10 tumors (five percent) showed 3+ staining in ≥50 percent of cells, while two tumors (one percent) showed 3+ staining in ≥10 percent but <50 percent of cells.12 Thus, if one decides to perform ISH on cases showing IHC 3+ staining in the 10 to 50 percent range, it would not appear overly burdensome (i.e. HER2 overexpression in colorectal cancer appears to be less heterogeneous than in gastroesophageal adenocarcinoma). Given less apparent heterogeneity in colon cancer and lack of clinical validation, I do not use the separate HER2 IHC biopsy criteria employed in gastroesophageal adenocarcinomas.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
