For syphilis screening, the central lab used the Bio-Rad BioPlex 2200 Syphilis IgG kit for detection of treponemal antibodies, and a syphilis RPR assay for detection of nontreponemal antibodies. If results were discordant, a syphilis TP-PA test was done, the results of which were definitive, Dr. Constantine said.
Eight hundred three samples were tested—324 from the University of Maryland and 479 from Fanno Creek Clinic. The 803 samples came from people known to be positive for HIV, known to be positive for syphilis, and known to be co-infected with HIV and syphilis, and from high-risk people of unknown status and low-risk individuals.
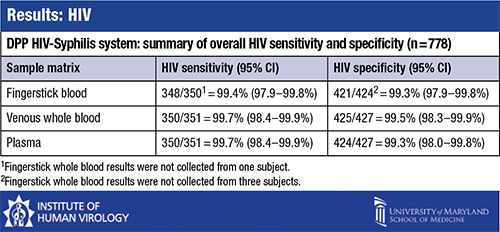
Reference test results found 351 samples to be positive for HIV, 119 to be positive for syphilis, and 91 to be co-infected. Four hundred twenty-seven were negative for HIV; 672 were negative for syphilis.
The comparison of the DPP HIV-Syphilis test results with the reference test results found that “for HIV, the test indices are about what you would expect and are excellent and sufficient, as most tests are for HIV,” Dr. Constantine said. For 778 samples, the HIV sensitivity was 99.4 percent for fingerstick blood and 99.7 percent for venous whole blood and plasma. Specificity was between 99.3 and 99.5 percent for fingerstick, whole blood, and plasma.
“For syphilis, sensitivity was a little less heavy depending on the matrix used, with fingerstick giving the lowest sensitivity,” at 93.3 percent, Dr. Constantine said. For venous whole blood the sensitivity was 96.6 percent; for plasma the sensitivity was 97.5 percent. Specificity was between 96.1 and 96.7 percent for all matrices. “If you average this out, it comes out to about 95 percent sensitivity for syphilis. Specificity is 96 percent or higher.”
Dr. Constantine suggested that seroreversion could have led to the false-negative TP-PA test results, “especially if a person is treated very early on during primary syphilis. Antibodies tend to not evolve as well, so that is responsible in some cases for false-negatives. The WHO also mentions that reason.” The false-negative results were found in patients who were previously diagnosed with syphilis. The degree of false-negatives between the DPP HIV-Syphilis system and the BioPlex 2200 Syphilis IgG kit was “essentially identical,” he said, at 79.4 percent.
In the syphilis reactive samples, there was good correlation between results from the DPP Micro Reader and the RPR titers.
What is the future of the DPP Micro Reader? “They tell me this is applicable,” he said, “for electronic transmission to smartphones, tablets, and other devices.”
Chembio is awaiting the FDA’s decision.
Amy Carpenter Aquino is CAP TODAY senior editor.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
