The consensus on three HER2-low breast cancer cases
Sherrie Rice
October 2022—HER2-low breast cancers are now of greater clinical interest, given Enhertu’s recent approval for use in treating such cancers. How to achieve accurate and reproducible results in scoring HER2-low tumors was at the center of a CAP TODAY webinar on new perspectives on the full spectrum of HER2 expression in breast cancer.

Dr. Tozbikian
“I think it may require increased training, increased operator competency, strict adherence to our ASCO/CAP scoring guidelines and criteria, taking more time with our cases, and optimizing our methods—using all our best practices,” said Gary Tozbikian, MD, webinar presenter and associate professor and director, Division of Breast Pathology, Department of Pathology, Ohio State University Wexner Medical Center. (The full webinar, sponsored by Daiichi Sankyo and AstraZeneca, is online at captodayonline.com.)
Of the 85 percent of breast cancers that are classified now as HER2 negative, about 60 percent will demonstrate some low level of HER2 expression, defined as having a HER2 IHC score of 1+ or 2+ without amplification (Schettini F, et al. NPJ Breast Cancer. Published online Jan. 4, 2021. doi:10.1038/s41523-020-00208-2). “And this 60 percent of patients with low levels of HER2 expression is a group of increasing clinical research interest,” Dr. Tozbikian said.
Currently, no formal definition exists for tumors with low levels of HER2 expression. However, most published studies that have looked at tumors with IHC expression of score 1+ or 2+ without HER2 ISH amplification have demonstrated that these cancers may have different clinical and pathologic features as compared with HER2-negative tumors with an IHC score of 0. For example, in a series of breast cancers with low levels of HER2 expression, Schettini, et al., found them to be more frequently hormone receptor positive than HER2 IHC 0 cancers—88 percent hormone receptor positivity versus 70 percent in those that are HER2 0 (Schettini F, et al. Ann Oncol. 2020;31[suppl 2]:S24). In that series, tumors with low levels of HER2 expression also showed significantly larger primary tumor size and more frequent nodal involvement, and PAM50 analysis showed differential gene expression with more frequent luminal expression profiles. In this study, Dr. Tozbikian said, there was no difference in grade, but in other studies these tumors were reported to show comparatively lower histologic grade compared with HER2 IHC score 0 tumors.
“So this subset of tumors may have some distinguishing biologic features,” he said. “But it is important to remember as a category, breast cancers with low levels of HER2 expression constitute a large number of tumors that demonstrate a wide degree of clinical and biologic heterogeneity.”
Further complicating the issue, he noted, is that intratumoral heterogeneity for HER2 expression is not uncommon. “It certainly adds to the interpretive challenge of HER2 evaluation and is a frequent cause of HER2 equivocal results.” Studies of HER2 nonamplified tumors with genomic heterogeneity have shown associations with larger tumor size, higher histologic grade, and frequency of lymph node metastasis (Marchiò C, et al. Semin Cancer Biol. 2021;72:123–135; Shafi H, et al. J Clin Pathol. 2013;66[8]:649–654), while in HER2-positive tumors there is published data that suggests intratumor heterogeneity for HER2 is associated with negative prognosis and lower pathologic complete response rates after neoadjuvant therapy (Filho OM, et al. Cancer Discov. 2021;11[10]:2474–2487; Lee HJ, et al. Am J Clin Pathol. 2014;142[6]:755–766).
 But, Dr. Tozbikian said, “The prognostic significance of low levels of expression is less well understood at this point.” A recent study found no significant prognostic differences between low HER2-expressing and HER2 IHC 0 breast cancers (Agostinetto E, et al. Cancers. 2021;13[11]:2824). The authors studied a data set from the The Cancer Genome Atlas—804 primary breast cancers, of which 410 were HER2 low. They observed no significant differences in overall survival or progression-free interval between HER2-low subtypes and each non-HER2-low subtype paired by hormone receptor status, though with a relatively short follow-up of under 28 months.
But, Dr. Tozbikian said, “The prognostic significance of low levels of expression is less well understood at this point.” A recent study found no significant prognostic differences between low HER2-expressing and HER2 IHC 0 breast cancers (Agostinetto E, et al. Cancers. 2021;13[11]:2824). The authors studied a data set from the The Cancer Genome Atlas—804 primary breast cancers, of which 410 were HER2 low. They observed no significant differences in overall survival or progression-free interval between HER2-low subtypes and each non-HER2-low subtype paired by hormone receptor status, though with a relatively short follow-up of under 28 months.
“The take-home point is that intratumoral heterogeneity is there. It certainly makes HER2 interpretation more challenging. It may impact prognosis. But the prognostic implication of low levels of HER2 expression as well as intratumoral heterogeneity for HER2 expression in these tumors deserves further study,” Dr. Tozbikian said.
Identifying cancers with low levels of HER2 expression will require high-quality, reproducible assays. Until they’re made available, Dr. Tozbikian said, the existing FDA-approved methods will have to be optimized, “controlling as best we can preanalytic and analytic variables, improving our operator competency, and adhering as strictly as possible to the ASCO/CAP HER2 IHC interpretation guidelines.” Other possibilities: using digital pathology and artificial intelligence for assisted scoring. “These things may be valuable,” Dr. Tozbikian said, “because the diagnostic and therapeutic landscape for HER2 continues to evolve and we as pathologists need to be prepared for that. We must adapt. In the meantime, pathologists may need to be more careful in examining the staining patterns of their cases to better discern score 0 from 1+ expression.”Optimal performance depends on multiple factors, he noted, each of which could take on greater importance when scoring tumors at the low end of the spectrum. The first is the type of specimen. For primary breast cancers, biomarkers are assessed for the most part on core needle biopsy specimens, which are not prone to prolonged cold ischemic time and other such issues. But they are subject to sampling error, “and this is an issue, especially if you’re dealing with a large tumor with potential intratumor heterogeneity for HER2 expression,” Dr. Tozbikian said. “So I think it remains to be seen whether biopsy versus resection is best for detecting HER2 expression at the low end of the spectrum.”
Core needle biopsies are prone to edge effects and crush artifact. “That limits interpretation.” At a minimum, he said, pathologists should be aware of and avoid scoring in cases that demonstrate artifacts.
Tissue site is a second variable, specifically as it pertains to the potential exposure of a specimen to decalcification solution, which is “highly relevant in breast cancer as bone metastasis is a common site that is sampled,” Dr. Tozbikian noted. Generally, decalcification reduces the antigenicity of the molecules targeted by IHC and is a potential cause of false-negative results. Breast biomarkers, including HER2, are not validated in decalcified specimens, and the presence of decalcification should at least be noted in a comment or be reason to flag a specimen as indeterminate as per ASCO/CAP guidelines, he advises.
A study published in 2016 examined the influence of decalcification procedures on IHC and molecular pathology in breast cancer (Schrijver WAME, et al. Mod Pathol. 2016;29[12]:1460–1470). “This study was interesting because they used more ‘gentle,’ more molecular-friendly solutions like EDTA and some formic-acid–based solutions,” Dr. Tozbikian said. They compared the impact of those treatments on ER, PR, and HER2 IHC in 23 prospectively collected breast tumors. For two patients (nine percent), there was a potential influence on therapeutic decision-making with regard to hormonal or HER2-targeted therapy. The negative impact of decalcification may be more pronounced in tumors with more subtle low levels of HER2 expression, Dr. Tozbikian said. “That’s something we need to be mindful of.”
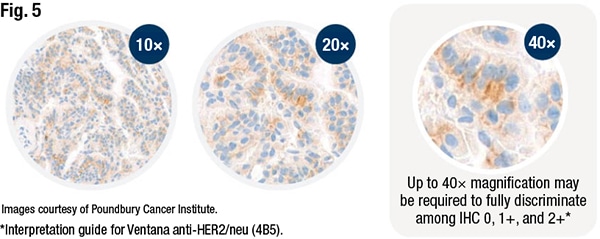 Specimen age, too, is a consideration. Where possible, he said, use freshly cut, unstained slides for HER2 assessment and try to avoid testing on archived, precut unstained slides, which can show staining degradation, due to cut sample instability. A 2004 study looked at the influence of slide aging on the results of translational research studies using IHC (Mirlacher M, et al. Mod Pathol. 2004;17[11]:1414–1420). The authors compared the staining intensity in a series of 522 breast cases using freshly cut unstained slides versus the archived slides that were six months old, and they observed that slide age had a negative impact on staining intensity. “Their HER2 positive rate decreased from 16.3 percent overall to 9.6 percent. And, as you can imagine, the potential impact on low levels of HER2 expression could be even more profound,” Dr. Tozbikian said.
Specimen age, too, is a consideration. Where possible, he said, use freshly cut, unstained slides for HER2 assessment and try to avoid testing on archived, precut unstained slides, which can show staining degradation, due to cut sample instability. A 2004 study looked at the influence of slide aging on the results of translational research studies using IHC (Mirlacher M, et al. Mod Pathol. 2004;17[11]:1414–1420). The authors compared the staining intensity in a series of 522 breast cases using freshly cut unstained slides versus the archived slides that were six months old, and they observed that slide age had a negative impact on staining intensity. “Their HER2 positive rate decreased from 16.3 percent overall to 9.6 percent. And, as you can imagine, the potential impact on low levels of HER2 expression could be even more profound,” Dr. Tozbikian said.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
