Latest on endocrinology, cardiology, pharmacology
Amy Carpenter Aquino
July 2023—The human microbiome has been called the forgotten organ, and at one time it was. But not in the past 10 years.
James Versalovic, MD, PhD, pathologist-in-chief and chair of pathology at Texas Children’s Hospital, made that clear in his talk at the Association for Molecular Pathology meeting last year.
For the human microbiome, “the past decade is akin to the 1990s and human genome research,” he said, “where we had to lay the road map. We had to construct the cartography of the human microbiome,” which began formally with the National Institutes of Health in 2007 but took root in 2012 with seminal publications on the NIH-supported Human Microbiome Project.
Since then, he said, “it’s been quite a ride.”
“I’m firmly convinced that in this decade and the next we will see the same amount of progress that we saw with human genome research in the last 20 years,” said Dr. Versalovic, the Milton J. Finegold professor and vice chair of pathology and immunology at Baylor College of Medicine. And it’s about more than genes. “It’s about cells,” he said. “It’s about communities of cells, similar to any organ in the human body.”
Dr. Versalovic is director of the Texas Children’s Microbiome Center. “Over the last 10 to 15 years,” he said, “we’ve discovered differences in metabolic pathways and the human gut microbiome in children versus adults.” Gut microbes in children may be producing more B-complex vitamins and folate. “Those pathways are enriched in children, and it may be that children with certain metabolic disorders have deficiencies in these pathways.” They’ve identified genes and pathways that could be used to address and correct deficiencies via oral supplementation with B-complex vitamins, for example (Hollister EB, et al. Microbiome. 2015;3:36).
Correlations are being established between the gut microbiome and human blood biochemistry, Dr. Versalovic said. “You’re going to be seeing more and more fusion of the two because many of the metabolites in human serum and plasma are derived from microbes in the gut and other body sites.”
Dr. Versalovic hit the highlights of microbiome research as they relate to endocrinology, cardiology, and pharmacology, beginning with microbial modulation of insulin resistance and type 2 diabetes.“Branched-chain amino acids—leucine, isoleucine, valine—are important in human metabolism,” he said (Fig. 1). These amino acids then feed the tricarboxylic acid (TCA) cycle, and oxidation of the amino acids can be implicated in different genetic disorders (Vanweert F, et al. Nutr Diabetes. 2022;12[1]:35).
This was brought to light, he said, with a study of 277 non-diabetic Danish adults for whom gut microbial data were available (Pedersen H, et al. Nature. 2016;535[7612]:376–381). The authors assessed the role of the gut microbiome as a source for key features of the serum metabolome profile, predicting metabolic and cardiovascular disorders in non-diabetic lean and obese people. They found these individuals to have elevated branched-chain amino acids that correlated with a state of insulin resistance and prediabetes, Dr. Versalovic said. “They also found that key organisms, namely Prevotella copri and Bacteroides vulgatus, correlated with elevations in branched-chain amino acids in human serum or plasma.”
P. copri and B. vulgatus are also the genes that encode for biosynthesis in branched-chain amino acids contributing to the metabolite pool, he said. Other microbes that may have contributed to elimination of branched-chain amino acids via specific transporters were deficient in the gut microbiome of insulin-resistant individuals. “So we begin to see the correlation between changes in the microbiome and potentially new biomarkers, such as specific amino acids that may augment known analytes such as hemoglobin A1c and serum blood glucose. In turn, new combinations of old and new biomarkers could improve the stratification of patients with insulin resistance, prediabetes, and ultimately type 2 diabetes,” Dr. Versalovic said. These findings may point in time to improved diagnostic testing and disease monitoring strategies for patients treated for type 2 diabetes.
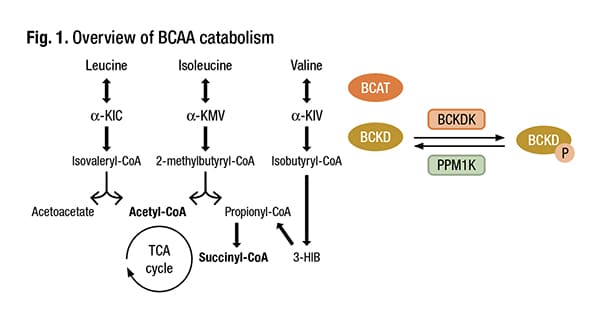
Schematic overview of BCAA catabolism. BCAT branched-chain amino acid transaminase, BCKD branched-chain keto acid dehydrogenase, α-KIC α-ketoisocaproate, α-KMV α-keto-methylvalerate, α-KIV α-ketoisovalerate, 3-HIB 3-hydroxyisobutyrate, BCKDK BCKDK kinase, PPM1K, BCKDK phosphatase. Adapted from Neinast et al.* Credit: Vanweert F, et al. Nutr Diabetes. 2022;12[1]:35. CC BY 4.0 License- https://creativecommons.org/licenses/by/4.0/ *Neinast M, et al. Annu Rev Physiol. 2019;81:139–164.
“There’s a real argument now for possibly adding measurements of branched-chain amino acids by mass spectrometry in the clinical laboratory to refine the stratification, assessment, and monitoring of patients who have or are at risk for type 2 diabetes,” Dr. Versalovic said. And it’s microbiome science that highlighted these findings. “What we’re learning about the microbiome is emphasizing potential pathways that may be important for diagnosis, monitoring, and ultimately treatment and prevention of different chronic disease states,” he said.
Intervention strategies to increase branched-chain amino acid oxidation and/or lower BCAA levels are important to investigate as a potential treatment for metabolic diseases, Vanweert, et al., write in their review (Vanweert F, et al. Nutr Diabetes. 2022;12[1]:35). There are key pathways, Dr. Versalovic said, in the oxidation of BCAAs in the human mitochondria. “And the rate-limiting step is the enzyme BCKDK,” or branched-chain ketoacid dehydrogenase kinase, which “can be blocked by particular pharmacologic inhibitors.” He cites as an example sodium phenylbutyrate, which has been used to treat patients who may have certain urea cycle disorders and maple syrup urine disease. The fibrates used in combination with statins to prevent cardiovascular disease also block this pathway. “With these drugs, we may be able to block BCKDK, the rate-limiting step, and that would result in decreased plasma branched-chain amino acid concentrations.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
