The education geared toward the activity menu will be aimed at eliminating confusion. “I think there is quite a bit of confusion now,” Dr. Karon says. “So we hope we’re going to clear up confusion about what labs should be doing about the PT and alternative performance assessment.”
“A lot of this is about education,” Dr. Bellizzi says of the activity menu and the educational program under study for interpretation-only laboratories. “We don’t want labs that are struggling with this class of testing to necessarily stop doing the testing. First we want to highlight that they’re doing this testing suboptimally, and then if it’s important to them to continue with the testing, we want to provide them resources to improve their testing.”
The needs of laboratories that stain and interpret differ from those for labs that only interpret, “and the next big push” will be to create products that meet the needs of the latter, he says. “Our preference is to create products so labs don’t have to think so hard about how to design alternative assessment.”
Knowing what PT or alternative performance assessment is required and following through is one thing. Addressing a failure is another, and failing to address a PT failure is important to avoiding a citation for a deficiency, says Harris S. Goodman, MD, chair of the CAP Checklists Committee, member of the CAP Council on Accreditation, and chief of the Department of Pathology, Alameda Health System Highland Hospital, Oakland, Calif. “One of the things I most commonly cite when I do inspections is when there’s been a PT failure and no one did anything about it,” he says. A laboratory can perform satisfactorily on a proficiency test event—get nine out of 10 challenges correct—but all failures have to be investigated.Laboratories need a procedure for addressing such failures, and most labs do, Dr. Goodman says. “It’s just that they don’t actually do it. So I come across a PT failure and I say, ‘How did you address this?’ And they say, ‘Well, it was only one out of 10, so we’re still in compliance.’ And I say, ‘But you still have to address the failure.’”
An investigation report is common in laboratories, he says, one that looks at the result obtained and the result expected, among other things. In his own laboratories, a checklist is used. “Was it a transcription error? Was the specimen not reconstituted properly? Not run properly? There’s a whole list of things we go through to see why it failed. And most labs have a procedure that says to do that. They just don’t necessarily do it.”
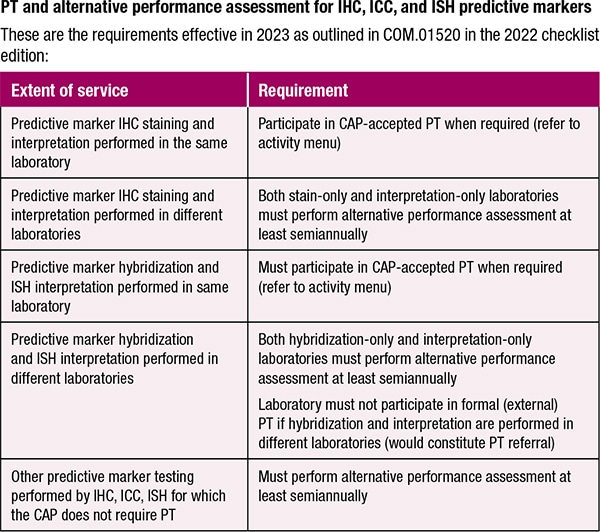
Dr. Goodman cautions, too, about inadvertent proficiency testing referral for laboratories that perform in situ hybridization. If the ISH and interpretation are performed in different laboratories, participation in formal PT would constitute PT referral. “Even if that’s the way patient specimens are handled, they are going to have to do an alternative performance assessment instead for ISH,” he says.
For predictive markers, he says, the requirements are method specific. He cites HER2 as an example. “If labs do different methods—IHC or FISH—to assess HER2 status, they have to participate in PT for both methods. They can’t just do one.”
For pathologists, predictive marker testing differs from other types of testing in which multiple characteristics are used to determine choice of therapy, Dr. Goodman notes. “With all these predictive markers, it’s just a single test, and so much rides on the single test, making it extremely important that we’re doing the test well.” Behind the CAP’s decision is its expectation that a lab perform its predictive marker testing well and use proficiency testing or alternative performance assessment to ensure it is performing well, and address problems if it’s not, he says.

Dr. Bellizzi
The CAP will offer webinars to guide labs in how to comply with what will be required, as well as a CAP22 session, “Tips and Tools for Quality Planning, Predictive Marker Monitoring, and Process Improvement in IHC,” led by Emily Meserve, MD, MPH, Russell Higgins, MD, and Dr. Bellizzi. In the 90-minute session to take place Oct. 9 on site, they will discuss the elements of an IHC laboratory quality plan, explain the requirements for the monitored IHC predictive markers, present case-based studies for process improvement of selected markers, and talk about strategies to be applied to additional markers, such as PD-L1, in the future.
The requirements for proficiency testing and alternative assessment for predictive markers are a way to “close the gaps so there’s a structure to hold accountable the labs that are doing this type of testing,” Dr. Bellizzi says. And the upcoming planned instruction will provide the assistance that some laboratories will need.
Limiting redundant Surveys enrollment is one benefit labs can expect to see, Dr. Karon says. “We’ll be reducing redundant proficiency testing for labs that might have had to enroll in five or six separate Surveys and that now may enroll in one per predictive marker.” And that is the goal as the number of markers requiring proficiency testing grows, he says: “to make a model that is not so burdensome for labs that they just can’t afford to do this anymore.”
Valerie Neff Newitt is a writer in Audubon, Pa. For a list of frequently asked questions from immunohistochemistry laboratories, and answers to those questions, go to https://bit.ly/CAP-IHC-FAQ.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
