Anne Paxton
May 2020—When tick-borne infections are the topic of discussion, talk of Lyme disease, caused by Borrelia burgdorferi, has tended to predominate. But as the prevalence of tick-borne infections in the United States rises steadily and widens geographically, the number of novel pathogens carried by ticks—including non-Lyme bacteria, protozoa, and viruses—has been climbing as well. The growing diversity of tick-borne pathogens is not only changing the conversation, but also posing an array of new challenges for clinical laboratories.
Bobbi Pritt, MD, MSc, DTM&H, chair of the CAP Microbiology Committee, has played a key role since 2011 in discovering and describing two previously unidentified tick-borne disease-causing bacteria, Ehrlichia muris eauclairensis and Borrelia mayonii. These particular species were infecting people not in New England, which once seemed the home base for tick-borne disease, but in the upper Midwest.
“Tick-borne diseases are progressively increasing every year throughout the United States and we’re seeing a number of different tick-borne diseases spreading,” says Dr. Pritt, director of Mayo Clinic’s clinical parasitology lab and co-director, vector-borne diseases laboratory services. Laboratory test volumes confirm the growth trend. One recent study, for example, found there was a 5.3-fold increase in testing for tick-borne infections and a threefold increase in positive results at Massachusetts General Hospital over the 11 years from 2006 to 2017 (Rudolf J, et al. Am J Clin Pathol. 2018;150[5]:415–420).
Increased testing for tick-borne diseases is a contributing factor in such rising numbers, Dr. Pritt and other experts suggest. But the ticks and the infections they carry are definitely on the increase and on the move. They are reaching from the upper Northeast down the east coast, outward from the upper Midwest through the black-legged deer tick (Ixodes scapularis).

Dr. Bobbi Pritt (left) and Dr. Elitza Theel at Mayo Clinic. “Tick-borne diseases are progressively increasing every year throughout the United States and we’re seeing a number of different tick-borne diseases spreading,” Dr. Pritt says.
Lesser known is the Lone Star tick, once confined to the South and Southeast but now found as far north as Wisconsin and parts of New England, Dr. Pritt notes. It does not transmit Lyme disease; it transmits ehrlichiosis and a number of other potentially fatal diseases, including a newly recognized meat allergy. In the western U.S., a tick called the Ixodes pacificus transmits Lyme and some other diseases along the Pacific Coast, while Dermacentor andersoni (Rocky Mountain wood tick) transmits Rocky Mountain spotted fever.
“We need to stop thinking of tick-borne illness as a very localized disease for an endemic area,” says Blake Buchan, PhD, D(ABMM), associate professor at the Medical College of Wisconsin and associate director of clinical microbiology at Wisconsin Diagnostic Laboratories. Several epidemiologic studies have confirmed the increasing range and geographic distribution of ticks carrying these pathogens over the past decade, he says. “It’s very important to recognize that these ticks don’t carry just Borrelia burgdorferi, but also other important pathogens.” More and more labs, he adds, “are recognizing the increased burden of tick-borne illnesses and the increased diversity of organisms that should be in the differential for patients displaying typical symptoms.”
“There are a lot of undiagnosed or misdiagnosed tick-borne infections out there due to the limitations of serologic methods and microscopic examination,” Dr. Buchan continues. “So when you have a positive Lyme serology result, you assume it’s Lyme and may not consider the possibility of acute infection with a different non-Lyme pathogen.” People should be aware of the potential for coinfections with two or more pathogens from one tick species, agrees Elitza S. Theel, PhD, who co-directs with Dr. Pritt the Mayo vector-borne diseases laboratory services.
Laboratories’ traditional use of serologic tests presents a twofold difficulty for purposes of detecting tick-borne diseases, Dr. Buchan says. “First, serologic tests don’t help in diagnosing acute infection because you have a window period before you seroconvert, before that IgM is detectable. With that low sensitivity, the tests don’t help people in the early stages of infection.”“As far as specificity, many people who are at risk for infection live in relatively endemic areas, so differentiating whether your symptoms are due to a recent infection or your serologic positivity is due to a past resolved infection can be challenging. With a molecular test, if you detect an organism directly, it’s definitive proof of an active infection. You can also pick this up prior to seropositivity, early in the infection during the acute phase when patients may be presenting.”

Dr. Buchan
Dr. Buchan and colleagues, in a recent article, provided an account of instances in which Borrelia burgdorferi was detected with a molecular test (Buchan BW, et al. J Clin Microbiol. 2019;57[11]:e00513-19). “We did that in nine whole blood specimens. None of them were seropositive. These were all acute infections that were completely missed because the patient had not seroconverted yet.” Likewise, he adds, “over 10 percent of the patients seropositive for Lyme were actually positive by the molecular test for a different, non-Lyme pathogen. So these would be misdiagnoses based on seropositivity of an old, resolved infection obscuring or being misleading about what is the current actual cause of your symptoms.”
Historically, Dr. Buchan says, “laboratories such as ours or other small labs in the U.S., maybe after doing a rapid blood smear and Lyme serologic test that’s negative, have sent out specimens for molecular testing at one of the reference labs. And that, of course, delays turnaround time and increases the costs of those results. The expanding commercial availability of reagents that can be used to develop your own laboratory-developed test may help to push this molecular testing into more regional or local high-complexity hospital laboratories. There, those reagents can be used on widely available platforms like the Applied Biosystems 7500 or the DiaSorin Liaison MDX, which are already installed in many labs.”
With the molecular tests, laboratories can say definitively not only which genus it is but also, in some cases, which species, he says. “We’ve seen Ehrlichia, which is historically thought not to be prevalent in Wisconsin because we don’t have the tick vectors. We’ve seen the emergence of a new Ehrlichia species, Ehrlichia muris, which is carried by our Ixodes tick.”
Unfortunately, as of yet there are no FDA-approved molecular tests for any tick-borne diseases, Dr. Pritt says. “Laboratories have to develop their own tests or bring in a test described in the literature and do extensive validation procedures before implementing the test in the laboratory.” Mayo Clinic has individual PCR assays for several non-Lyme diseases, and Dr. Pritt and Dr. Theel have worked with Mayo clinicians to develop an algorithm that labs can follow in using the assays. “There are a lot of different tests out there,” says Dr. Pritt. “You don’t want to just go to the lab catalog and order every test listed.”
Instead, she says, the laboratory needs to ask: “Where in the country are you? What tick-borne diseases are in your area? And then use an algorithmic approach to evaluate for the different diseases.” As the Mayo algorithm shows (this page), some tests should be ordered reflexively in sequence. “Or there may be situations where no testing is indicated.”
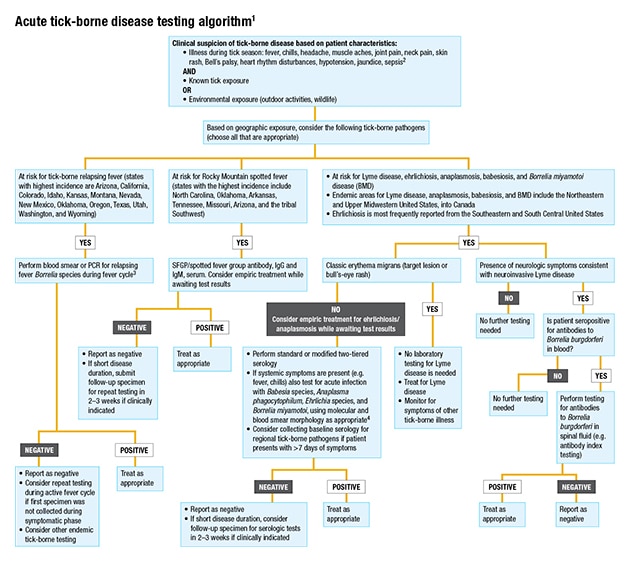
1 Covers testing for the most common tick-borne pathogens in the United States. Not all inclusive.
2 In the presence of severe neurologic symptoms, contact public health authorities for additional testing options (e.g. Powassan/deer tick virus, Heartland virus, Bourbon virus).
3 If infection is thought to have been acquired outside the Americas, order both blood smear and PCR. Not all species of relapsing fever Borrelia may be detectable by a single PCR.
4 Local test availability may vary. PCR testing of blood may be useful for detection of Borrelia mayonii in patients with exposure to ticks in Minnesota or Wisconsin.
The general guidance, Dr. Theel says, when a patient presents with acute symptoms such as fever and myalgias, alongside an appropriate exposure history, is to perform molecular testing for everything except Lyme disease within the first seven days of symptom onset. From eight days after symptom onset, “we then recommend serologic testing be performed, because by that point the immune response has had time to develop and otherwise immunocompetent people will typically have detectable antibodies to the organism.”
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
