Shahla Masood, MD
Anwer Siddiqi, MD, MS
August 2023—We discuss in this article a common problem that all cytopathologists come across frequently in their practice: tumors of unknown primary origin involving body fluids and other sites.
Metastatic tumor cells can disseminate and colonize discontinuous secondary body sites.1 Such tumor metastases may be the patient’s initial presenting complaint to a family physician for deep-seated tumor primaries such as ovaries, pancreas, liver, and certain non-obstructive gastrointestinal tumors. These tumors may metastasize to the pleural, peritoneal, and pericardial cavities and to lymph nodes, other organ systems, brain, and fibroconnective tissue including bone and subcutaneous tissue, presenting as ascites, pleural effusion, cardiac tamponade, or palpable nodules (enlarged lymph nodes), among other things. Cytologic evaluation of the samples from the preceding sites may result in the initial cancer diagnosis. However, further clinical workup may be required to determine the primary site of origin and to confirm the initial cytologic cancer diagnosis
Pleural, peritoneal, or pericardial effusions could be a complication of advanced malignancy with significant morbidity and mortality. The most common cancer metastatic to the pleural cavity in men and women is lung adenocarcinoma, followed by mammary carcinoma in women. A small number of lung adenocarcinomas may also involve the pericardial cavity. The gastrointestinal adenocarcinomas, including pancreatobiliary tumors, may involve pleural or peritoneal cavities. The majority of ovarian malignancies involve the peritoneal cavity, although a good number may also present with pleural effusions.
The first step in evaluating a cytologic specimen with no known primary is characterizing the cytomorphologic features of the cells. What is the cell arrangement: single cell versus two-dimensional sheets or three-dimensional clusters with acinar formation? Do the cells have an epithelial, plasmacytoid, epithelioid, or spindled appearance? Are the cells in question mesothelial or histiocytic? Also important to know is if the cytoplasm is ample or scant, if there are prominent nucleoli, and if the chromatin is coarse or salt and pepper.
Once the answers become available, then based on the sex of the patient and any other relevant findings in the clinical history, physical exam, or lab results, one can come up with a differential diagnosis, often followed with several immunostains to interrogate the cells of interest to help confirm a diagnosis. In difficult cases with no clear direction based on the pattern of immunostains, listing the top differentials in the report and recommending further clinical workup are essential to get to the final diagnosis. Keep in mind that insisting on a specific diagnosis in the absence of corroborative clinical and imaging findings may be misleading.
At our institution we follow a flow chart (Fig. 1) to work up malignancies of unknown primary sites, once we determine the basic cell type (epithelial/mesenchymal), to further characterize the lineage of tumor cells in the absence of a prior history of malignancy.
However, there are cases in which, despite all our efforts, we are able only to report metastatic carcinoma (AE1/AE3 positive). In these cases, the immunostains provide specific direction, and the results still have to be reported cautiously with a disclaimer suggesting correlation with the subsequent clinical and imaging findings.
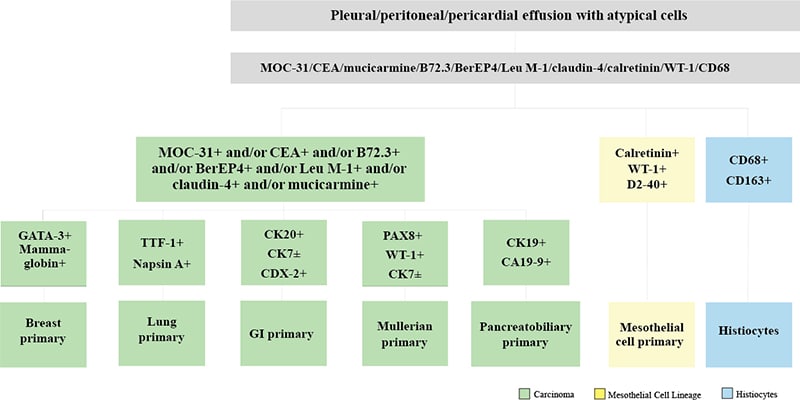
Fig. 1. Malignant effusion cytology workup for the primary
The first step of working up tumors in body fluids is to rule out that the cells in question are not reactive mesothelial cells or malignant mesothelioma. The reactive mesothelial cells can easily be mistaken for cancer cells. There is a long list of available immunostains and special stains that could be used selectively depending on the situation to differentiate between mesothelial and epithelial cells. They include calretinin, WT-1, D2-40, CEA, claudin-4, B72.3, MOC-31, Leu-M1, and mucicarmine. If it’s pleural or pericardial effusion, lung primary (Fig. 2) has to be ruled out using TTF-1 or p40 depending on the tumor cell cytomorphology. In the case of a female patient, breast primary (GATA3) (Fig. 3) needs to be ruled out. Occasionally, we have made a diagnosis of breast cancer on pleural effusions and following our diagnosis a breast mass was subsequently discovered. We cannot stress enough the importance of a thorough physical exam, including breast exam and imaging.
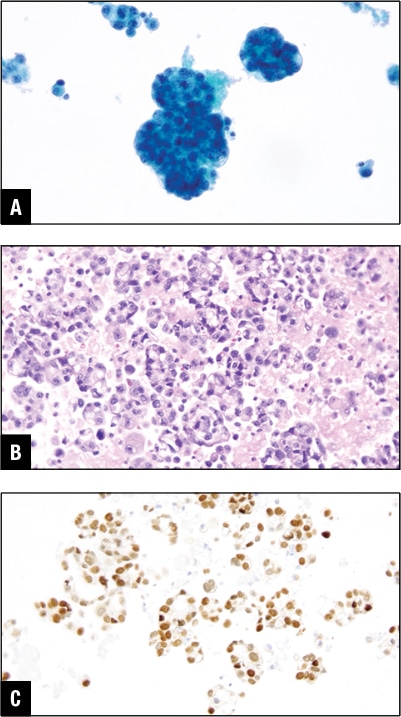
Fig. 2. Lung adenocarcinoma in pleural effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. TTF-1 positive in tumor cells.
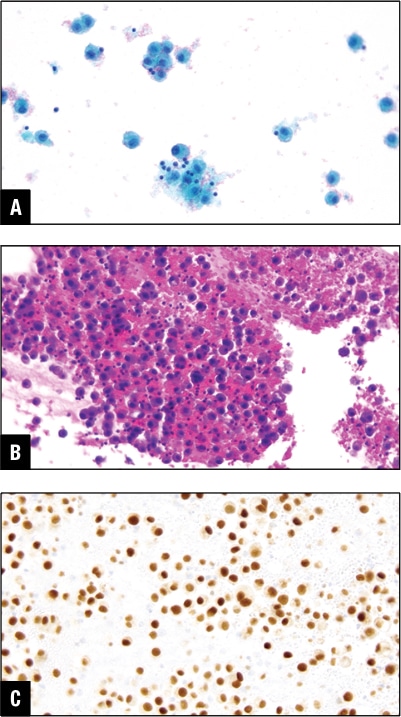
Fig. 3. Mammary carcinoma in pleural effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. GATA3 positive in tumor cells.
During the workup, if the stains for lung and breast are negative and there is no doubt the cells are epithelial, then we must broaden our search and use a CK7 and CK20 combination to look for other differentials for the primary. In real practice, instead of waiting for CK7 and CK20 results, the pathologist may order CDX2, PAX8, or other stains, depending on their experience and observation, to get to the diagnosis. Waiting to check the results of CK7 and CK20 before ordering subsequent stains may be economical, but the downside is that cutting into a block multiple times may result in tissue exhaustion. Every effort should be made to conserve tissue for possible molecular studies. Also, one should avoid ordering unnecessary stains to minimize patient expense; that will also conserve tissue. However, the pathologist’s primary focus is correct diagnosis. Economy, tissue conservation, and medicolegal concerns are secondary.
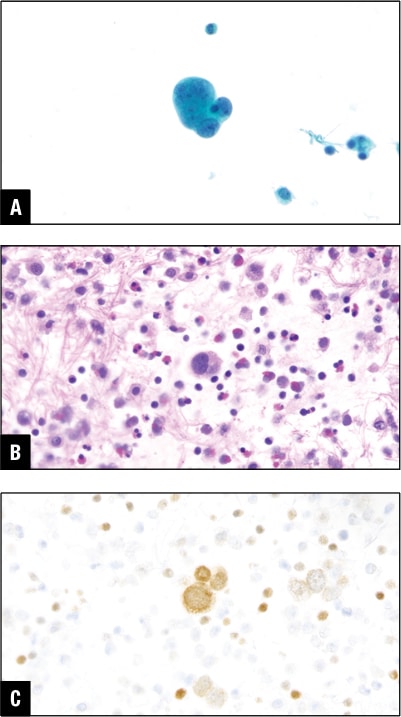
Fig. 4. Urothelial carcinoma in peritoneal effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. GATA3 positive in tumor cells.
We mostly use immunostains to confirm our provisional diagnosis, but rarely we use immunostains like a compass to give us direction, especially in poorly differentiated malignancies and in the absence of clinical and imaging studies. In such circumstances one should keep an open mind. Two years ago, a 50-year-old man presented to UF-Jacksonville with malignant ascites with no known prior history of malignancy. The malignant cells stained positive for GATA3 and he was eventually diagnosed with a urothelial primary (Fig. 4).
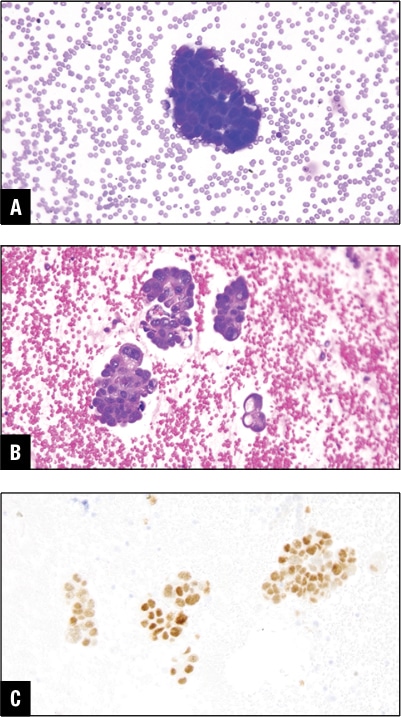
Fig. 5. Ovarian carcinoma in peritoneal effusion. A. Tumor cells in DiffQuik stain. B. Tumor cells in H&E cell block section. C. PAX8 positive in tumor cells.
PAX8 and/or WT-1 positive malignant cells in ascitic fluid point toward Mullerian primary in women. In our practice this is one of the common causes of developing malignant peritoneal effusion (Fig. 5).
Among the other common primaries for malignant peritoneal effusions are gastrointestinal tract malignancies including colon (Fig. 6) and the pancreatobiliary primary (Fig. 7). These may give rise to peritoneal or pleural effusion, and some may even involve the pericardial cavity.
Rarely the abnormal effusion fluid cells may stain with mesothelial cell markers like calretinin, WT-1, and D2-40. The malignant mesothelioma cells are larger than normal mesothelial cells, and even if you see clusters of 12 cells or more, even though the cells do not appear atypical, entertaining malignant mesothelioma in the differential is a wise choice. The final diagnosis will depend on clinical, imaging correlation, and other studies.2
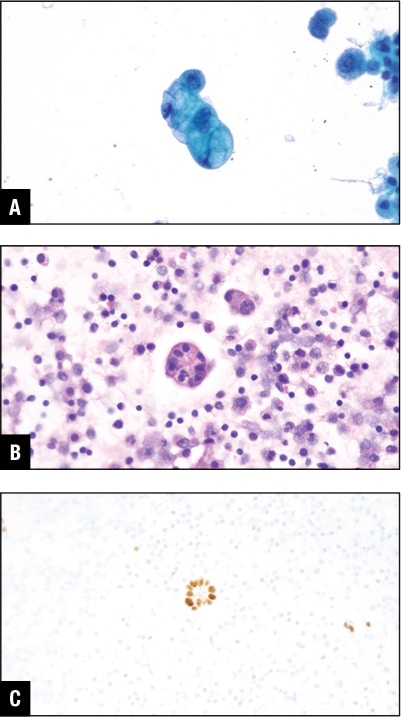
Fig. 6. Colon adenocarcinoma in peritoneal effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. GATA3 positive in tumor cells.
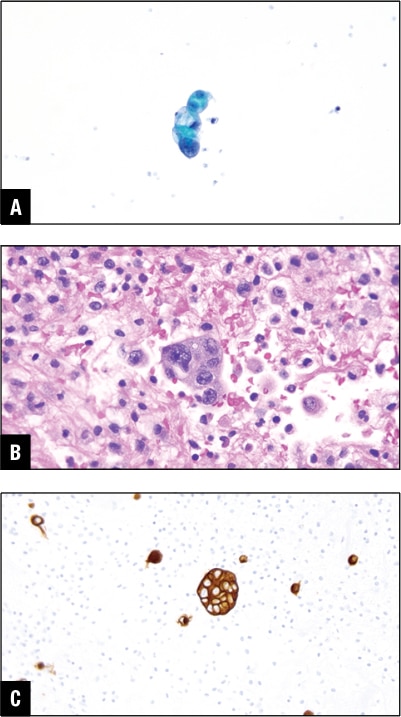
Fig. 7. Pancreatic ductal carcinoma in peritoneal effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. CA19-9 positive in tumor cells.
An immunocompromised individual presented with pleural effusion; the cells are shown in Fig. 8. The initial impression based on the cytomorphologic features was adenocarcinoma, but the stains for lung, other carcinomas, as well as mesothelial markers all came back negative. The patient did have pigmented skin lesions, but the biopsy was inconclusive. Finally, melanoma markers were tried and all were positive. Always remember malignant melanoma is the great masquerader.
Presence of numerous histiocytes (CD68 and CD163+) in effusion fluids could be due to irritation of the serosal surfaces and is referred to as nodular histiocytic/mesothelial hyperplasia. This is a reactive process and should not be confused with malignancy.2-4
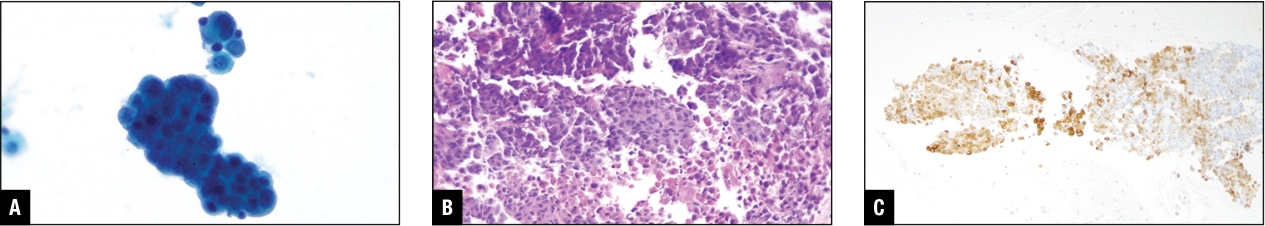
Fig. 8. Malignant melanoma in pleural effusion. A. Tumor cells in Pap stain. B. Tumor cells in H&E cell block section. C. Melan-A positive in tumor cells.
For the sake of completeness, we should also mention malignant effusions due to hematopoietic malignancies. This would require a separate review; in some non-Hodgkin’s lymphomas a part of the cytology specimen should be sent for flow cytometry and for definitive diagnosis. For other lymphomas, B and T cell markers, CD15, CD30, ALK, and kappa/lambda in situ hybridization on the cell block will clinch the diagnosis.
- Welch DR, Hurst DR. Defining the hallmarks of metastasis. Cancer Res. 2019;79(12):3011–3027.
- Cibas ES, Ducatman BS. Cytology: Diagnostic Principles and Correlates. 5th ed. Elsevier; 2020.
- Churg A, Cagle PT, Roggli VL. Tumors of the Serosal Membranes. American Registry of Pathology; 2006. Atlas of Tumor Pathology; series 4.
- Choi YL, Song SY. Cytologic clue of so-called nodular histiocytic hyperplasia of the pleura. Diagn Cytopathol. 2001;24(4):256–259.
Dr. Masood is professor and chair of the Department of Pathology and Laboratory Medicine, University of Florida College of Medicine, Jacksonville, and a member of the CAP Cytopathology Committee. Dr. Siddiqi is associate professor at the University of Florida College of Medicine.
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
