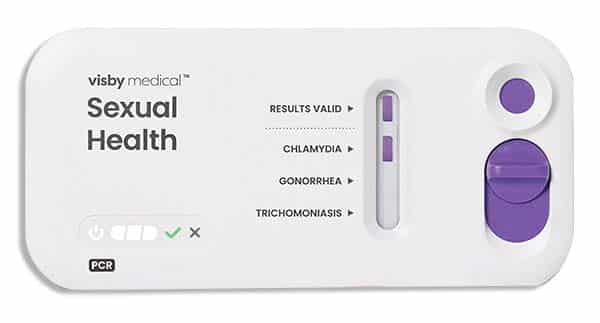
 April 2023—Visby Medical has received 510(k) clearance and was granted a CLIA waiver from the FDA for its second-generation Sexual Health test. The point-of-care test uses PCR technology to detect sexually transmitted infections caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Visby says the new device features improvements in workflow, manufacturability, and reliability. Test accuracy is about 97 percent and results are available in less than 30 minutes.
April 2023—Visby Medical has received 510(k) clearance and was granted a CLIA waiver from the FDA for its second-generation Sexual Health test. The point-of-care test uses PCR technology to detect sexually transmitted infections caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Visby says the new device features improvements in workflow, manufacturability, and reliability. Test accuracy is about 97 percent and results are available in less than 30 minutes.
Visby Medical, 833-468-4729
Pages: 1 2
 CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
