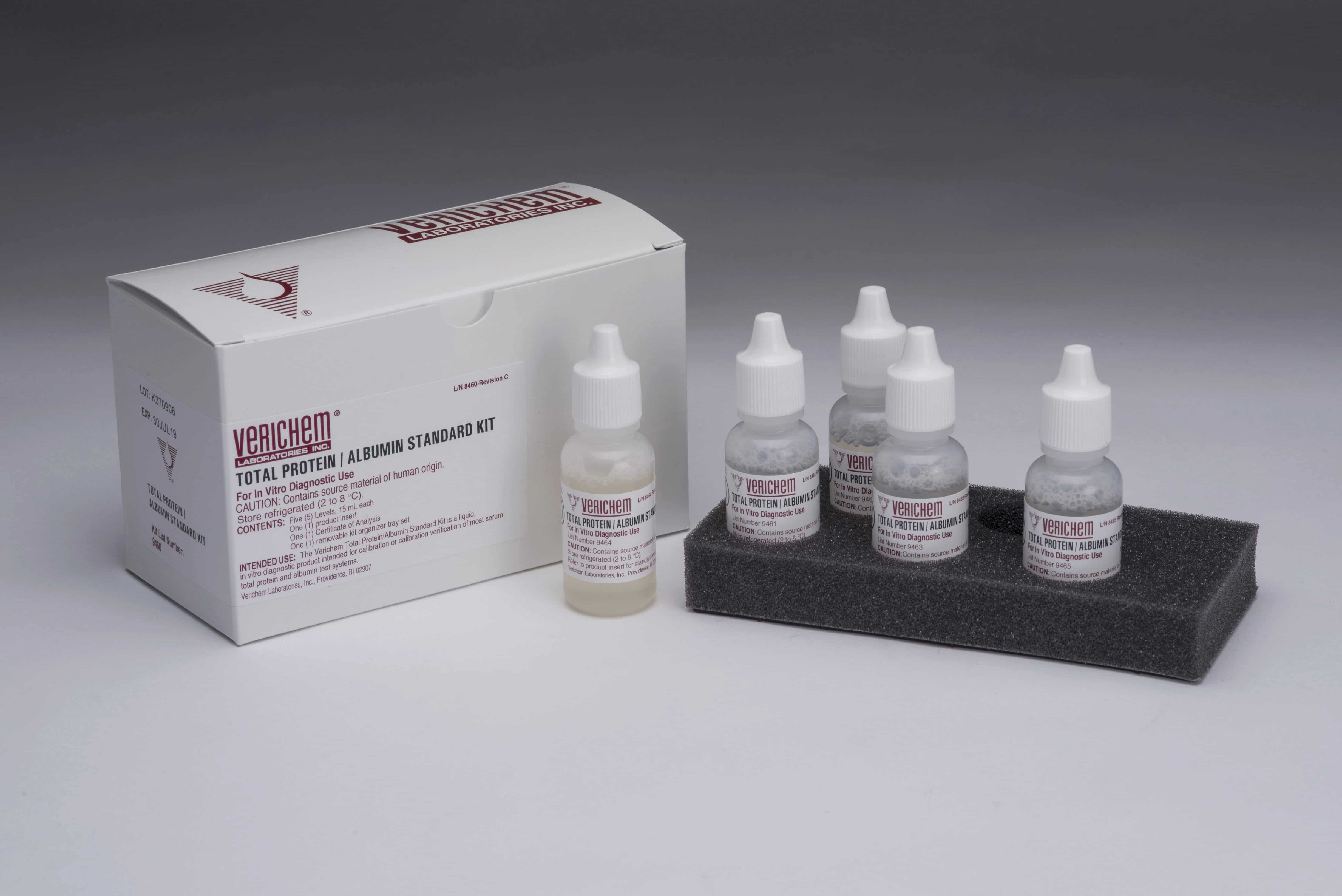
April 2024—Verichem Laboratories offers a multilevel set of clinical reference materials intended for the calibration verification of clinical systems testing for total protein and albumin in urine and cerebrospinal fluid samples. The liquid-stable and ready-to-use materials are suitable for use with turbidimetric and colorimetric test methods and incorporate human protein components.
Verichem reference materials for creatinine assays
March 2024—The Matrix Plus chemistry reference kit and standalone, ultra-high Matrix Plus level F for the calibration verification testing of creatinine assays by enzymatic and colorimetric methods are available from Verichem Laboratories. The ready-to-use reference materials offer constant protein content and pH across all concentration levels. The five-level Matrix Plus kit and level F feature a creatinine range from 0.2 mg/dL to 30.0 mg/dL and are intended to be treated as patient specimens. Shelf life and open-vial stability are 21 months from the manufacturing date.
Read More »Verichem bilirubin, urine chemistry reference materials
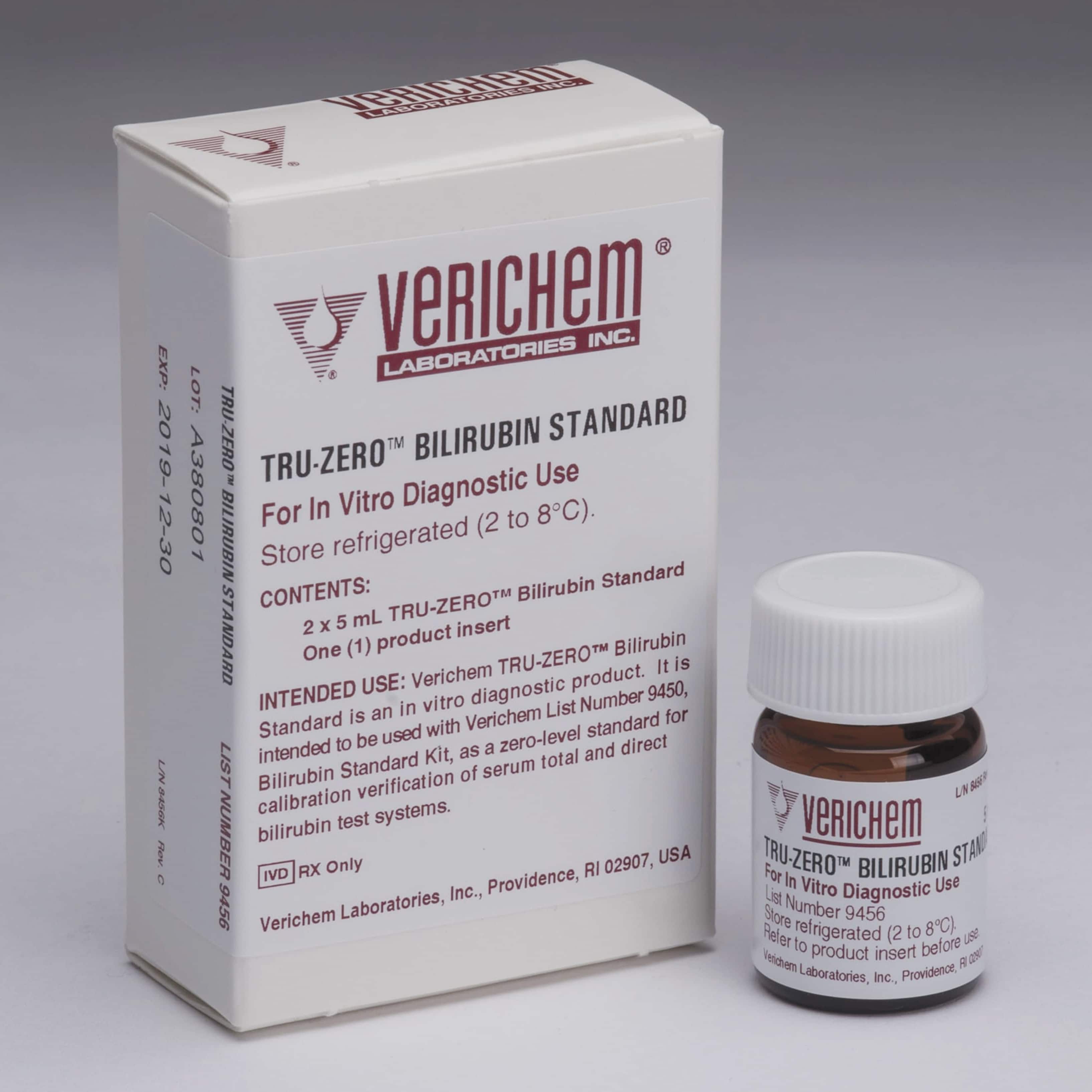
February 2024—Verichem Laboratories now offers its Tru-Zero Bilirubin Standard as part of the company’s line of liquid-stable and protein-based total and direct bilirubin clinical reference materials. The standard is intended to be treated as a patient specimen and features universal instrument compatibility. The concentration level for the total and direct bilirubin assays is 0.0 mg/dL. The product has an open-vial stability claim of five days and a shelf life of 14 months when stored at 2° to 8°C.
Read More »Verichem reference materials for serum and urine testing
November 2023—Verichem Laboratories announced the availability of its Urine Uric Acid Standard kit, Matrix Plus Cholesterol Reference kit, and a standalone, ultra-high Matrix Plus Cholesterol Reference level F for the calibration verification testing of cholesterol and urine and serum uric acid assays. The Urine Uric Acid Standard kit is a five-level kit with uric acid concentrations ranging from 1 to 101 mg/dL. The materials feature universal testing compatibility and are composed of a biosynthetic matrix with urine-like activity. Storage temperature is −15° to −25°C and Verichem says the product can tolerate up to 10 freeze-thaw cycles with no effect on accuracy or performance. Shelf life is 19 months.
Read More »Verichem enzyme calibration verifiers for liver function testing
October 2023—Verichem Laboratories offers multilevel enzyme calibration verification materials for liver function testing as part of its liquid-stable Enzyme ER Verifier kit. The kit contains the enzymes alanine aminotransferase (ALT/SGPT), alkaline phosphatase (AP/ALP), aspartate aminotransferase (AST/SGOT), gamma-glutamyl transferase (GGT/GGTP), lactate dehydrogenase (LD/LDH), amylase, cholinesterase, creatine phosphokinase, and lipase. Its proprietary formulation is designed to include at least one concentration for each enzyme within the normal range. The verifiers are intended to be treated as patient specimens and are compatible with wet chemistry systems from Abbott, Beckman Coulter, Roche, and Siemens Healthineers. Shelf life is 18 months.
Read More »Verichem reference materials for electrolyte testing
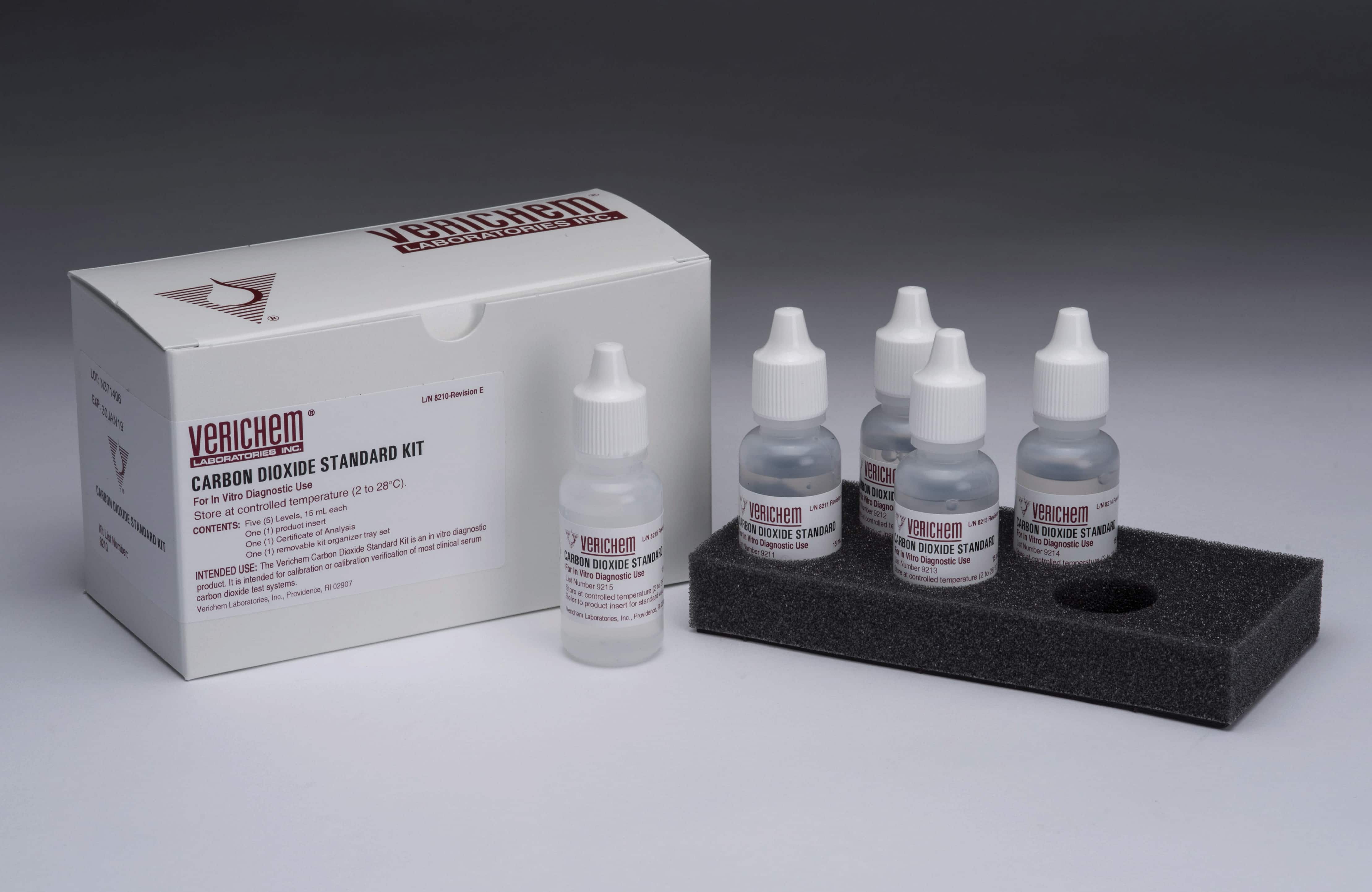
September 2023—Verichem Laboratories offers a line of liquid-stable, multianalyte kits for the calibration verification of electrolyte assays for use with systems employing flame photometer, direct or indirect ion-selective electrode, or standard enzymatic/colorimetric testing methods. The Electrolyte Standard kit contains the analytes chloride, ionized calcium, lithium, potassium, and sodium and has a 21-month stability claim. The ISE Standard kit contains chloride, lithium, potassium, and sodium and has an 18-month stability claim. The Carbon Dioxide Standard kit and standalone CO2 Standard level F both have 15-month stability claims. All products are free of azides, glycols, surfactants, and other potentially interfering substances.
Read More »Verichem calibration verification reference materials
August 2023—A multilevel set of liquid-stable clinical reference materials for use with ammonia and iron testing are available from Verichem Laboratories. The five-level standard kit, along with an optional, standalone ultra-high sixth level, is designed for the calibration verification of ammonia and iron assays on a range of automated clinical systems, including from Abbott Diagnostics, Beckman Coulter, Roche Diagnostics, and Siemens Healthineers. The materials are treated as patient specimens and free of glycols, surfactants, azides, and other interfering substances. Iron concentration levels of 10–1,000 μg/dL are verified using standard reference materials from the National Institute of Standards and Technology, and ammonia concentrations of 10–2000 μg/dL are verified using reagent-grade standards from the American Chemical Society. Shelf life is 24 months.
Read More »Verichem offers free data reduction reports
July 2023—Verichem Laboratories now offers its customers free access to its Web-based and online calibration verification data reduction and test-reporting programs. Designed for use with the company’s clinical reference materials, these programs and services provide the necessary statistical data and test reports to satisfy CLIA requirements for the calibration verification of clinical assays. Users can register online with a password, log in, and then enter and submit data; there is no software to download. Reports are generated instantly in a PDF format and are ready to review, sign off, and file. Final reports can include clinical system test accuracy, linearity, precision, precision with peer comparison, and other supplemental study data. Technical support is available via phone to answer questions or troubleshoot.
Read More »Verichem calibration verifiers for cholinesterase testing
June 2023—Verichem Laboratories offers multilevel calibration verification materials for cholinesterase activity as part of its liquid-stable and ready-to-use Enzyme ER Verifier kit. The verifiers are designed to include at least one concentration level of enzyme activity within the normal range and intended to be treated as patient specimens. The material’s protein balance, pH, and ion content are constant across concentration levels. The kit material also contains multilevel assigned activities for alanine aminotransferase, alkaline phosphatase, alpha-amylase, aspartate aminotransferase, creatine phosphokinase, gamma-glutamyl transferase, lactate dehydrogenase, and lipase. Each kit has 5 mL of material for each of the six levels and a shelf-life and open-vial stability of 18 months.
Read More »Verichem calibration verifiers, reference materials
May 2023—Verichem Laboratories now offers multilevel calibration verification materials for amylase and lipase activity as part of its liquid-stable, ready-to-use Enzyme ER Verifier kit. The product is for use on automated chemistry systems and has a shelf life of 18 months. The kit contains one 5-mL dropper vial for each of the six levels.
Read More »Verichem reference materials for triglyceride assays
April 2023—Verichem Laboratories released ready-to-use reference materials for use with calibration verification testing of triglyceride assays. The product is available in a five-level standard kit and an optional, standalone, ultra-high sixth level, with levels ranging from 10 to 1,250 mg/dL. Tricglyceride values are established by gravimetric weight of glycerol expressed as triolein.
Read More »Verichem Matrix Plus chemistry reference materials
April 2024—Verichem Laboratories liquid-stable Matrix Plus chemistry reference materials for the calibration verification of wet chemistry assays are now available. The multilevel kit, along with an optional and standalone level F, supports overall system quality control and CLIA compliance. The six-level set of materials contains seven individual chemistry analyte components covering 48 separate and individual concentration levels.
Read More »Verichem Enzyme ER Calibration Verifier kit
March 2023—Verichem Laboratories’ ready-to-use Enzyme ER Calibration Verifier kit is now available. The materials are designed for the calibration verification of enzyme assays on wet chemistry testing systems and contain nine clinical enzyme components covering 54 individual activity levels. The enzyme components within the materials include amylase, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, cholinesterase, creatine phosphokinase, gamma glutamyltransferase, lactate dehydrogenase, and lipase. The materials are intended to be treated as patient specimens and have a shelf life of 18 months.
Read More »Verichem reference materials for toxicology testing
March 2023—Verichem Laboratories announced the availability of ready-to-use, multilevel clinical reference materials intended for use with toxicology assays on a range of wet chemistry analyzers.
Read More »Verichem reference materials for cholesterol assays
January 2023—Verichem Laboratories offers liquid-stable, ready-to-use clinical reference materials intended for calibration and calibration verification procedures for total cholesterol and high- and low-density lipoprotein assays.
Read More »Verichem reference materials for lactate assays
December 2022—Verichem Laboratories announced the availability of a six-level, protein-based kit designed for the calibration or calibration verification of lactate assays on automated clinical systems. The liquid-stable, ready-to-use product contains concentration levels of lactate ranging from 3.2 mg/dL to 160 mg/dL. The format is free of glycols, surfactants, azides, and other interfering substances. Shelf life is 18 months from the manufacturing date.
Read More »Verichem reference materials for ethanol testing
November 2022—Verichem Laboratories announced the availability of a ready-to-use set of multilevel clinical reference materials for the calibration and calibration verification of ethanol assays on a range of diagnostic testing systems. The protein-based liquid standards are free of surfactants, glycols, azides, and other interfering substances. Each kit contains 5 mL of material per vial at the six available concentration levels and has a shelf life of 18 months when stored at 2° to 8°C. The product also contains a known linear relation for acetaminophen, lactate, and salicylate.
Read More »Verichem reference materials for protein assays
October 2022—Verichem Laboratories offers liquid-stable, ready-to-use clinical reference materials for protein testing. The multilevel and combined Total Protein/Albumin Standard Kit, along with the optional and standalone level F, and the Microprotein Standard Kit are designed and manufactured for the calibration and calibration verification of albumin and total protein assays on a range of clinical testing systems, including from Abbott Diagnostics, Beckman Coulter, Roche Diagnostics, and Siemens Healthineers.
Read More »Reference materials for urine chemistry, bilirubin assays
August 2022—Verichem Laboratories now offers a range of liquid-stable and ready-to-use biosynthetic clinical reference materials for use with urine chemistry assays and designed for system calibration and calibration verification testing on a variety of clinical testing platforms, including from Abbott, Beckman Coulter, Roche, and Siemens Healthineers.
Read More »Verichem reference materials for ethanol, total protein, albumin
July 2022—Verichem Laboratories announced the availability of clinical reference materials intended for the calibration or calibration verification of ethanol assays.
Read More »Verichem reference materials for ammonia
June 2022—Verichem Laboratories announced the availability of a multilevel set of liquid-stable, ready-to-use clinical reference materials for the calibration and calibration verification of ammonia assays. The materials are universally compatible and can be used with a range of clinical testing systems, including from Abbott Diagnostics, Beckman Coulter, Roche Diagnostics, and Siemens Healthineers.
Read More »Clinical reference materials for cholesterol, electrolytes
February 2022—Verichem Laboratories offers an array of liquid-stable, ready-to-use multilevel reference materials for total cholesterol, high-density lipoprotein, and low-density lipoprotein testing. The Matrix Plus Total Cholesterol Reference Kit, the Matrix Plus Total Cholesterol Reference Level F, and the HDL Cholesterol Verifier Kit are intended for calibration verification and are compatible with a variety of wet chemistry clinical analyzers.
Read More »Verichem reference materials for microprotein testing
November 2021—Verichem Laboratories offers ready-to-use, liquid-stable clinical reference materials for microprotein testing, suitable for use with turbidimetric and colorimetric test methods. The products are intended for the calibration and calibration verification of clinical systems testing for total protein and albumin in urine and cerebral spinal fluid. The kit contains a five-level set with 15 mL of material at each level and provides 10 individual certified concentrations in an azide-free format, incorporating human protein components for optimum reactivity.
Read More »Verichem carbon dioxide reference materials
September 2021—Verichem Laboratories offers a selection of ready-to-use carbon dioxide reference materials. The five-level standard kit along with an optional level F are intended for calibration and/or calibration verification of clinical systems and are suited for use with ion-selective electrode and wet testing methodologies.
Read More »Reference materials for bilirubin, cholesterol, uric acid
August 2021—Verichem Laboratories offers liquid-stable, protein-based clinical reference materials for total and direct bilirubin assays. The Bilirubin Standard Kit, Tru-Zero Bilirubin Standard, and extended-range Level F Bilirubin Standard are intended for CLIA calibration verification with wet chemistry testing systems. Verichem’s BR2 Bilirubin Calibrator is available for use exclusively with the BR2 Bilirubin Stat Analyzer from Advanced Instruments.
Read More »Verichem Labs reference materials
July 2021—Verichem Laboratories announced the availability of ready-to-use, liquid-stable clinical reference materials for the calibration and calibration verification of clinical testing systems.
Read More »Bilirubin calibrator, standard kits
February 2021—Verichem Laboratories released a liquid-stable, protein-based BR2 Bilirubin Calibrator designed for the calibration of Advanced Instruments’ Advanced BR2 Bilirubin Stat Analyzer. The product has an open-vial stability claim of five days and an unopened shelf-life claim of 14 months at 2° to 8°C. The bilirubin calibrator is packaged in amber serum vials with rubber-lined closures and contains 5.0 mL of standard material, with two vials per kit.
Read More »Verichem Labs offers data reduction services
January 2021—Verichem Laboratories is offering its customers free access to the company’s online, web-based data reduction services. Designed for use with Verichem’s products, the service supports the necessary statistics required within the clinical laboratory to satisfy current CLIA and CAP requirements for the calibration verification of clinical assays.
Read More »Verichem Labs reference materials
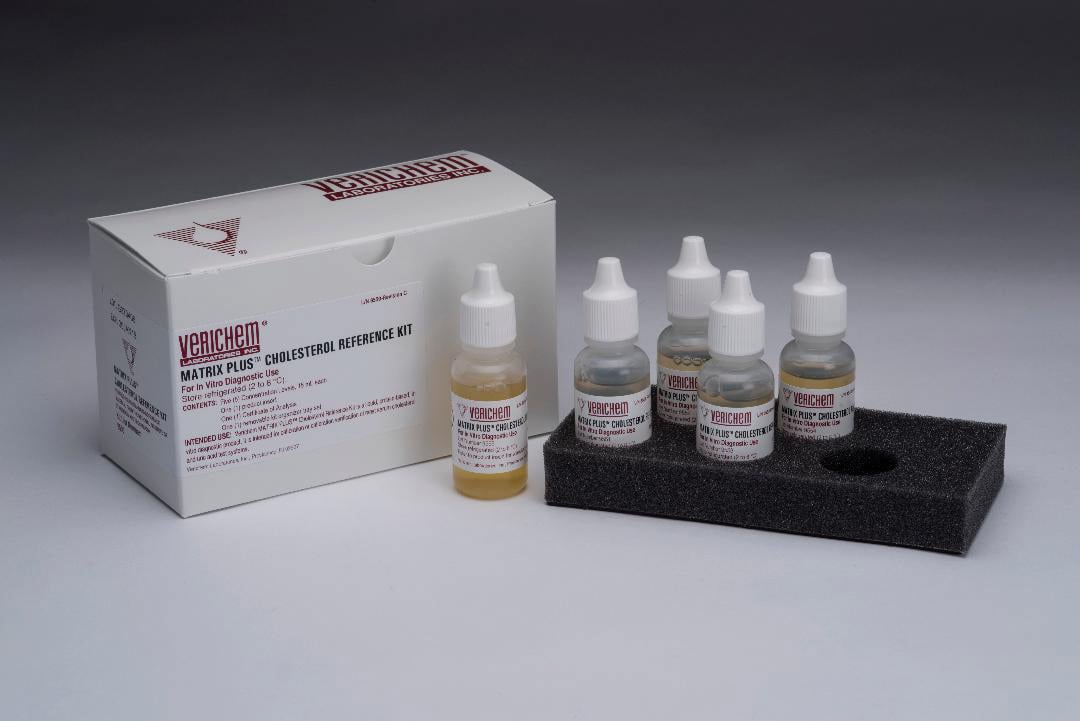
December 2020—Protein-based, ready-to-use liquid Matrix Plus Cholesterol Reference kits are available from Verichem Laboratories. The kits are designed to support overall system quality control and CLIA compliance and are for the calibration or calibration verification of cholesterol and uric acid wet assays on clinical testing systems. The materials contain esterified and free cholesterol from bovine serum. The standard kit with the sixth level F covers 12 concentration points; cholesterol concentrations range from 40 to 750 mg/dL and uric acid concentrations from 2 to 30 mg/dL. Active components are verified using standard reference materials from the National Institute of Standards and Technology. Shelf life is 21 months from the date of manufacture. Ready-to-use, liquid Total Protein and Albumin reference materials are also available. The combined Total Protein/Albumin Standard kit, along with the optional standalone Total Protein/Albumin Standard level F, are designed for the calibration and calibration verification of albumin and total protein assays on a wide number of clinical testing systems. The standards are prepared with human serum albumin and gamma-globulin serum proteins in a saline-based solution. Shelf life is 24 months from the manufacturing date.
Read More »Verichem standards kits
October 2020—A ready-to-use microprotein standard kit is available from Verichem Laboratories. The kit is intended for the calibration verification of total protein and albumin concentrations in urine and cerebral spinal fluid. The five-level set provides 10 certified concentrations in an azide-free liquid format. The kit is suitable for use with turbidimetric and colorimetric testing methods and incorporates human protein components. Shelf life is 24 months from the manufacturing date when stored at 2° to 8°C.
Read More »Verichem calibration verification kits, standards
August 2020—Verichem Laboratories announced the availability of its Enzyme ER Verifier Kit designed for the calibration verification of wet chemistry testing systems. The multianalyte, six-level kit of liquid stable materials is composed of nine clinical enzyme components—amylase, alanine aminotransferase, alkaline phosphatase, aspartate aminotransferase, cholinesterase, creatinine kinase, gamma-glutamyl transferase, lactate dehydrogenase, and lipase—covering 54 activities.
Read More »Verichem standard kits for ethanol, urine uric acid
March 2020—Verichem Laboratories announced the availability of its ethanol standard kits. The protein-based, liquid standards are intended for calibration or calibration verification of serum ethanol test systems. The product also contains lactate, salicylate, and acetaminophen in a known linear relation and may be used to determine the linearity, sensitivity, and reportable range for these analytes as well. The reference materials are free of surfactants, glycols, azide, and other interfering substances. The kit contains a six-level set with 5.0 mL of each level.
Read More »Verichem calibration verification kits
February 2020—Verichem Laboratories announced the availability of its HDL Cholesterol Verifier kit intended for the calibration verification of HDL and LDL cholesterol assays. The kit contains high-density and low-density lipoprotein cholesterol from human serum in a ready-to-use, liquid stable format that is free of surfactants, glycols, and azide.
Read More » CAP TODAY Pathology/Laboratory Medicine/Laboratory Management
CAP TODAY Pathology/Laboratory Medicine/Laboratory Management